Prescribing Principles
Antimicrobial Prescribing Principles
Antimicrobial Prescribing Principles
- Doses of antimicrobials are for non-obese ADULTS with normal renal and liver function.
- GUH have agreement to use the Children's Health Ireland (CHI) Antimicrobial Guidelines for patients less than 18 years old. The guidelines are available as part of the CHI Paediatric Formulary app ( Clinibee ). You may be asked to register an account using your email address. The lead contact is Dr. Edina Moylett.
- When antimicrobial treatment is appropriate the prescriber should ensure that there is a clear clinical justification documented in the patient’s notes .
- When required, samples for culture (blood, sputum, pus etc as appropriate) should be taken prior to commencing or changing antimicrobials unless the patient’s condition necessitates immediate initiation of treatment.
- Review antimicrobial therapy at least daily in the context of clinical progress and culture and other laboratory results. If a pathogen(s) is identified or no pathogen is identified, use the result as appropriate to guide therapy.
- For all patients who identify themselves or who are otherwise identified as penicillin allergic establish the relevant history, assess and document. See Penicillin Hypersensitivity .
- Prescribers should be aware of contraindications, warnings, precautions, interactions and potential adverse effects of all drugs prescribed, including antimicrobial agents. These are outlined in the Summary of Product Characteristics (SPC) (available at HPRA ) and BNF.
- Access GUH Intravenous Medicines Administration Guide for additional information on the safe prescription and administration of individual intravenous antimicrobials.
- See Interactions Appendix 1 for online interaction checkers.
- See Antimicrobial Prescribing in Renal impairment for dose adjustment.
- Switch IV to oral as soon as it is appropriate to do so. See IV to PO switch therapy .
- Stop antimicrobials as soon as it is appropriate to do so based on guidance on duration of treatment and clinical response.
- HSE Information for patients on antibiotics including possible side effects can be found at https://www2.hse.ie/conditions/antibiotics/
- See Fluoroquinolone warning
Fluoroquinolones
Systemic fluoroquinolones are associated with disabling and potentially permanent serious side-effects involving the tendons, muscles, joints, nerves, and aorta. The risk of tendinopathy is increased in elderly patients – or those with concomitant steroid use, renal disease, or post-transplant (heart/lung/kidney). Fluoroquinolone use is also associated with QTc prolongation (which can lead to torsades de pointes and ventricular fibrillation), C. difficile colitis, aortic aneurysm rupture/dissections and heart valve regurgitation/ incompetence. See SPC for full product information. Patients should be informed of the risks and advised to stop treatment and contact prescriber if they experience pain or swelling in tendons/joints/muscle or neuropathy.
Dosing in Obesity
- The number of obese patients is increasing and the standard doses of some drugs may not achieve effective serum concentrations. Data on antimicrobial dosing in the obese patient are gradually emerging, but only some drugs have been evaluated. Contact Pharmacy /Micro /ID / re optimising antimicrobial dose in obese patients.
Understanding Antimicrobial Susceptibility Test Reports
- These guidelines are primarily intended to guide empirical treatment of infection. If a specific pathogen is identified and susceptibility test results are available you should generally change from an empirical approach to a laboratory guided approach to treatment. The following is a brief guide to using reported susceptibility test results. These results are not a perfect guide and must always be considered in the overall clinical context. Discuss with Microbiology or Infectious Disease as required.
- Organism is reported as resistant to an antimicrobial agent. Do not use this agent.
- Organism is reported as susceptible to an antimicrobial agent. This agent is likely to be effective at the standard dose and frequency of administration in the context of other necessary patient care measures (such as source control and hydration)
- Organism is reported as susceptible increased exposure (SIE). This agent is likely to be effective provided dose and frequency of administration are appropriate to achieve adequate levels and in the context of other necessary patient care measures (such as source control and hydration)
Note Regarding Multi-drug Resistant Organisms (MDRO)
MDRO general information
- MDRO are organisms exhibiting resistance to more than one group of antimicrobials. They include Gram-negative organisms such as extended-spectrum beta-lactamase (ESBL)-producing bacteria and carbapenemase-producing Enterobacterales (CPE), and Gram-positive organisms such as methicillin resistant Staphylococcus aureus (MRSA) and vancomycin resistant Enterococci (VRE).
- Check with the patient and the patients records to determine if they are already known to be colonised with one or more MDRO or if they require testing for colonisation with MDRO.
-
Patients at identifiable increased risk for colonisation or infection with MDRO include:
- Those with prior or prolonged hospitalisation.
- Residents of long term care facilities.
- Those exposed to multiple antimicrobials, especially broad spectrum antimicrobials.
- Those with indwelling medical devices, particularly urinary catheters.
- Recent travel to countries where MDROs are more common, in particular if they have received healthcare in those countries.
- Discuss with Microbiology or Infectious Diseases if patient suspected or known to be colonised with MDRO as alternative treatment or surgical prophylaxis regimen may be required.
Refs:
1. National Clinical Effectiveness Committees Guideline No. 30 Infection Prevention and Control https://www.gov.ie/en/publication/a057e-infection-prevention-and-control-ipc/
Note Regarding Carbapenemase-Producing Organisms (CPO) and Carbapenemase Producing Enterobacterales (CPE)
- CPO are Gram-negative bacteria that carry genes for resistance to carbapenems (e.g. meropenem). They are often resistant to carbapenems and to many other antimicrobial agents.
- CPE are the most common subset of CPO. CPE are mostly K. pneumoniae, E. coli , and Enterobacter spp. but other species of Enterobacterales may also have this mechanism of resistance.
- Most patients who are colonised with CPE are identified from routine testing of rectal swabs or faeces. Colonisation means the organism is present but is not associated with infection.
- There is no antimicrobial treatment that has been shown to be useful in clearing gut colonisation with CPE or other CPO. There is good reason to believe that giving antimicrobial treatment to colonised patients supports persistent colonisation.
- When patients colonised with CPE or other CPO develop infection it may be caused by the CPE or by other organisms. If the patient is seriously ill the initial empiric treatment may need to cover for the CPE they are colonised with. Treatment options in cases of infection with CPE are often limited. If a patient with CPE from a rectal screen and/or clinical sample develops clinical evidence of an infection seek advice on antimicrobial therapy from Microbiology or Infectious Diseases as appropriate.
Refs:
- National Clinical Effectiveness Committees Guideline No. 30 Infection Prevention and Control https://www.gov.ie/en/publication/a057e-infection-prevention-and-control-ipc/
- Treatment of suspected or confirmed infection with Enterobacterales or Acinetobacter spp. Resistant to carbapenems. Surgical prophylaxis in the context of colonization with such organisms. a-guide-to-treatment-of-infection-with-carbapenem-resistant-organism-april-2019.pdf (hse.ie)
Note Regarding Extended-Spectrum Beta-Lactamase (ESBL) producing bacteria
- ESBL are Gram-negative bacteria that produce beta-lactamase enzymes capable of inactivating a wide range of beta-lactam antimicrobial agents. This usually includes most penicillins and cephalosporins. The ESBL species most commonly associated with infection are E. coli and K. pneumoniae .
- Colonisation with ESBL organisms is now very common in Ireland. Although ESBL colonisation and infection are more common in patients with identifiable risk factors (see above list for MDRO), colonisation has been reported in otherwise healthy members of the general population.
- There is no antimicrobial treatment that has been shown to be useful in clearing gut colonisation with ESBL. There is good reason to believe that giving antimicrobial treatment to colonised patients supports persistent colonisation.
- Most ESBL remain susceptible to nitrofurantoin and fosfomycin which can be effective for treatment of uncomplicated cystitis caused by ESBL.
- For those with complicated urinary tract infection or infection at other sites many ESBL remain susceptible to piperacillin/tazobactam, gentamicin and restricted agents such as meropenem.
- ESBL colonisation is most common in those with extensive healthcare exposure including acute hospitals and long-term residential care facilities for older people. Empiric cover for ESBL blood stream infection with Meropenem should be considered in patients admitted from nursing homes who are critically ill with sepsis . Discuss with Microbiology or Infectious Diseases as required.
Note Regarding Vancomycin Resistant Enterococcus (VRE)
- Enterococcus faecium is naturally resistant to many antimicrobial agents. Vancomycin is one of a limited number of agents available for treatment of serious infection with Enterococcus faecium. Gut colonisation with E. faecium that has acquired resistance to vancomycin is now very common in patients with extensive healthcare exposure.
- There is no antimicrobial treatment that has been shown to be useful in clearing gut colonisation with VRE. There is good reason to believe that giving antimicrobial treatment to colonised patients supports persistent colonisation.
- For patients with serious/life threatening infection who are at risk for VRE infection, empiric treatment with linezolid or daptomycin is generally indicated in addition to the other components of therapy recommended in this guideline. Discuss with Microbiology or Infectious Diseases as required.
Note Regarding Methicillin Resistant Staphylococcus aureus (MRSA)
For infection at almost any site you should suspect infection with MRSA if:
- Patient has been previously colonised with MRSA.
- Patient has recently been hospitalised (within 90 days).
- Patient has transferred from another hospital or long-term care facility.
- Patient is on a ward with a current epidemic or endemic MRSA problem.
For patients with serious/life threatening infection who are at risk for MRSA infection, empiric treatment with Vancomycin is indicated in addition to the other components of therapy recommended in this guideline. Discuss with Microbiology or Infectious Diseases as required.
Documentation of Antimicrobial Use
Documentation of Antimicrobial Use
Accurate documentation is a key component of appropriate antimicrobial prescribing. It improves communication between medical, nursing and pharmacy staff and between different medical practitioners who may review therapy throughout the prescribed course and subsequently. It also facilitates multidisciplinary audit of antimicrobial prescribing within and between hospitals, to inform and improve education and action plans to improve antimicrobial practices.
Key elements to consider and document when prescribing antimicrobials are:
R - Route : Please review all IV antimicrobials DAILY
I - Indication for the antimicrobial e.g. pneumonia
D - Duration/Review Date e.g. 7 days for hospital acquired pneumonia
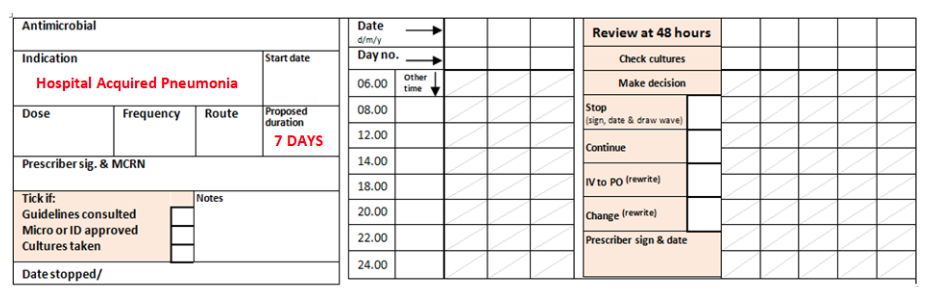
Always document the treatment indication and treatment duration or review date in both the appropriate box in the antimicrobial section of the drug chart (example shown below) AND in the patient’s medical notes.
Example of antimicrobial section of drug chart with indication and duration documented
Topical Antibacterial Agents
|
Topical Antibacterial Agents |
||
|
Not routinely recommended |
Rationale for restricting use in hospitals |
Exceptions |
|
Any topical antibacterial e.g. Bactroban ® Flamazine ® Fucidin ® Naseptin ® Polyfax ®
|
Emergence of resistance
An infection that needs to be treated should generally be treated systemically |
|