Adult Guidelines
Abdomen
Abdomen
- The regimens below may NOT cover Multi-drug Resistant Organisms (MRDO) in all cases. See note on MDRO .
- Fungal Infection is an important consideration in patients with intra-abdominal sepsis. In patients at high risk of fungal infection e.g. upper GI perforation, consider antifungal therapy; discuss with Microbiology or Infectious Diseases.
- Most patients with acute pancreatitis do NOT have necrotising pancreatitis and do NOT require antibiotic prophylaxis.
|
Empiric Antibiotics for Abdominal Infections |
||||||
|
Infection |
1 st Line Antibiotics |
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
Comment |
||
|
See penicillin hypersensitivity section for further information |
||||||
|
The regimens below may NOT cover Multi-drug Resistant Organisms (MRDO) in all cases. See note on MDRO |
||||||
|
Intra-abdominal Mild Community Acquired e.g. cholecystitis/ appendicitis/ diverticulitis |
Co-amoxiclav IV 1.2g every 8 hours |
CefUROXime IV 1.5g every 8 hours + Metronidazole** IV 500mg every 8 hours |
Ciprofloxacin** IV 400mg every 12 hours + Metronidazole** IV 500mg every 8 hours |
Duration 4 to 7 days assuming adequate source control |
||
|
Intra-abdominal Moderate to Severe Community & All Hospital Acquired including cholangitis/ intra-abdominal abscess/diverticulitis |
Piperacillin/ tazobactam IV 4.5g every 8 hours Add Gentamicin IV IF sepsis . Give one dose per GAPP App calculator. See footnote* re further doses and monitoring. |
CefTRIAXone IV 2g every 24 hours + Metronidazole** IV 500mg every 8 hours Add Gentamicin IV IF sepsis . Give one dose per GAPP App calculator. See footnote* re further doses and monitoring. |
Ciprofloxacin** IV 400mg every 12 hours + Gentamicin IV one dose per GAPP App calculator. See footnote* re further doses and monitoring. + Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re monitoring. + Metronidazole** IV 500mg every 8 hours |
Discuss with Microbio-logy or Infectious Diseases. Duration 7 to 10 days assuming adequate source control. |
||
|
Infected Necrotising Pancreatitis Patients with acute pancreatitis admitted to ICU or necrotising pancreatitis confirmed by imaging |
CefTRIAXone IV 2g every 24 hours + Metronidazole IV 500mg every 8 hours |
Ciprofloxacin IV 400mg every 12 hours + Metronidazole IV 500mg every 8 hours |
Review need for antibiotics every 72 hours. See note below. |
|||
|
Discuss with Microbiology or Infectious Diseases if deterioration or requiring antibiotics for more than 5 days |
||||||
|
Spontaneous Bacterial Peritonitis |
CefTRIAXone IV 2g every 24 hours Add Gentamicin IV IF sepsis. Give one dose per GAPP App calculator. See footnote* re further doses and monitoring. |
Ciprofloxacin** IV 400mg every 12 hours Add Gentamicin IV IF sepsis. Give one dose per GAPP App calculator. See footnote* re further doses and monitoring. |
5 days |
|||
|
Peritoneal Dialysis Peritonitis |
Vancomycin Intraperitoneally 30mg/kg (max. 3g) loading dose, then 30mg/kg (max. 2g) every 5 to 7 days + Ciprofloxacin PO 500mg every 12 hours
|
|||||
|
Cirrhosis with Acute Variceal Haemorrhage, Prophylaxis |
CefTRIAXone IV 2g every 24 hours
|
Ciprofloxacin PO 500mg every 12 hours |
7 Days |
|||
|
Prophylaxis for patients with an absent or dysfunctional spleen |
Phenoxymethyl-penicillin PO 666mg (Calvepen ® )
every 12 hours
Amoxicillin PO 500mg every 24 hours |
Erythromycin PO 250 to 500mg every 24 hours |
Oral absorption of phenoxymethylpenicillin is limited and affected by a number of variables. For emergency self initiated therapy of a suspected systemic infection treatment with amoxicillin is preferable. See Appendix 2 for guidelines for management of patients with absent or dysfunctional spleen (adults only) including recommended vaccines & antibiotics. |
|||
|
Emergency treatment doses |
||||||
|
Amoxicillin PO 500mg to 1g every 8 hours |
Erythromycin PO 500mg to 1g every 6 hours |
|||||
|
* Review need for ongoing Gentamicin and Vancomycin on a daily basis. Continue with once daily Gentamicin dosing ONLY if Consultant/Specialist Registrar recommended. For advice on monitoring see Gentamicin & Vancomycin Dosing & Monitoring section. **Switch from IV to oral Ciprofloxacin and Metronidazole as soon as possible |
||||||
Refs:
- IDSA Guidelines for Diagnosis and Management of Complicated Intra-abdominal infections in Adults & Children. Clin Infect Dis 2010;50:133-164 (Archived)
- The Surgical Infection Society revised guidelines on the management of intra-abdominal infection. Surg Infect 2017:18:1.
- American Gastroenterological Association clinical practice update: management of pancreatic necrosis. Gastroenterology 2020;158:67-75
- 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg 2019:14:27.
- BMJ Best Practice: Spontaneous bacterial peritonitis. Updated 23 rd Oct 2023. Bestpractice.bmj.com/topics/en-gb/793
- ISPD Peritonitis guideline recommendations:2022 update on prevention and treatment. Perit Dial Int 2022;42(2):110-153.
- Prophylactic antibiotics on patients with cirrhosis and upper gastrointestinal bleeding: a meta-analysis. PLoS ONE 17(12):e0279496.
- GUH Procedure for Treating a Patient with Peritonitis (QPulse CLN-NM-095)
Bone and Joint
Bone and Joint
- Discuss all cases with Microbiology or Infectious Diseases.
- Microbiological diagnosis is essential , relevant microbiological samples (bone/synovial fluid AND blood cultures) should be sent prior to treatment.
- In patients with sepsis, antibiotic therapy should not be delayed while awaiting bone or synovial fluid sampling.
- The regimens below may NOT cover Multi-drug Resistant Organisms (MDRO) in all cases. See note on MDRO .
|
Empiric Antibiotics for Bone and Joint Infections |
|||||
|
Infection |
1 st Line Antibiotics |
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
Comment |
|
|
See penicillin hypersensitivity section for further information |
|||||
|
The regimens below may NOT cover Multi-drug Resistant Organisms (MRDO) in all cases. See note on MDRO |
|||||
|
Native joint Septic Arthritis Nil risk factors for MRSA |
Flucloxacillin IV 2g every 6 hours
|
CeFAZOlin IV 2g every 8 hours |
Vancomycin IV infusion, dose per GAPP App Calculator. See footnote* re monitoring. |
Adequate drainage of joint fluid essential. Discuss all cases with Microbiology or Infectious Diseases. |
|
|
Known colonisation/risk factors for MRSA |
Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re monitoring. |
||||
|
Risk of gonococcal infection OR High risk of Gram-negative organisms (Elderly, Nursing home resident, recurrent UTI) |
CefTRIAXone IV 2g every 24 hours |
Discuss with Microbiology or Infectious Diseases |
|||
|
Osteomyelitis |
Discuss with Microbiology or Infectious Diseases; treat according to culture results. |
Short course oral antibiotic therapy may be appropriate for acute on chronic osteomyelitis |
|||
|
Prosthetic Joint Infection |
Discuss with Microbiology or Infectious Diseases; treat according to culture results. |
||||
|
*For advice on monitoring see Vancomycin Dosing & Monitoring section. |
|||||
Ref:
-
BSR & BHPR, BOA, RCGP and BSAC guidelines for management of the hot swollen joint in adults Rheumatology 2006;45:1039-1041
-
Ravn C, Neyt J, Benito N, Abreu MA, Achermann Y, Bozhkova S, Coorevits L, Ferrari MC, Gammelsrud KW, Gerlach UJ, Giannitsioti E, Gottliebsen M, Jørgensen NP, Madjarevic T, Marais L, Menon A, Moojen DJ, Pääkkönen M, Pokorn M, Pérez-Prieto D, Renz N, Saavedra-Lozano J, Sabater-Martos M, Sendi P, Tevell S, Vogely C, Soriano A, The Sanjo Guideline Group. Guideline for management of septic arthritis in native joints (SANJO). J Bone Jt Infect. 2023
-
Éric Senneville, Zaina Albalawi, Suzanne A van Asten, Zulfiqarali G Abbas, Geneve Allison, Javier Aragón-Sánchez, John M Embil, Lawrence A Lavery, Majdi Alhasan, Orhan Oz, Ilker Uçkay, Vilma Urbančič-Rovan, Zhang-Rong Xu, Edgar J G Peters, IWGDF/IDSA Guidelines on the Diagnosis and Treatment of Diabetes-related Foot Infections (IWGDF/IDSA 2023), Clinical Infectious Diseases, 2023;, ciad527, https://doi.org/10.1093/cid/ciad52 7
Cardiovascular
Bacterial Endocarditis
Bacterial Endocarditis
- The following is intended primarily to provide initial short-term guidance on empiric therapy of seriously ill patients and those with prosthetic valves.
- Immediate consultation on the next day with Microbiology or Infectious Diseases is recommended in all cases of suspected endocarditis.
- In those with sub-acute presentation of suspected endocarditis, with a native valve and who are clinically stable at presentation it is often preferable to send blood cultures (as below) and to withhold antibiotics pending consultation and culture results.
- In all but the most profoundly ill patients take 3 sets of blood cultures (10ml into each of two bottles for each set) BEFORE any antibiotics are given. If the patient is seriously ill the interval between cultures can be as short as 20 or 30 minutes.
|
Empiric Antibiotics for Bacterial Endocarditis |
|||
|
Infection |
1 st Line Antibiotics
|
Penicillin allergy: immediate or severe delayed reaction |
Comment |
|
See penicillin hypersensitivity section for further information |
|||
|
Bacterial Endocarditis |
Native Valve or Prosthetic valve Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re monitoring. + Gentamicin IV 1mg/kg (maximum 80mg) every 12 hours. See footnote* re monitoring + CefTRIAXone IV 2g every 24 hours
|
Native Valve or Prosthetic valve Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re monitoring. + Gentamicin IV 1mg/kg (maximum 80mg) every 12 hours. See footnote* re monitoring + Ciprofloxacin PO 500mg every 12 hours (IV 400mg every 12 hours if NPO)
|
Once culture and sensitivity results are available direct antibiotic therapy accordingly in discussion with Microbiology or Infectious Diseases.
Duration as advised by Microbiology or Infectious Diseases.
|
|
* For advice on monitoring see Gentamicin & Vancomycin Dosing & Monitoring section. |
|||
Refs:
- Gould et al. BSAC Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults. Journal of Antimicrobial Chemotherapy 2012;67:269-289
- Baddour et al. AHA Infective Endocarditis: Diagnosis, Antimicrobial Therapy, and Management of Complications. Circulation 2015;132:1435-1486
- Delgado et al. ESC Guidelines for the management of endocarditis: Developed by the task force on the management of endocarditis of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). European Heart Journal , 2023 ; 44: 3948–4042
Prophylaxis of Infective Endocarditis
Prophylaxis of Infective Endocarditis
- The routine use of antibiotics in most situations is NOT justified on the balance of risk and benefit.
- Consult with Microbiology or Infectious Diseases recommended if infection at procedure site.
- Only patients identified with the following cardiac conditions undergoing one of the following high risk procedures should be considered for prophylaxis for infective endocarditis (IE):
|
Prophylaxis of Infective Endocarditis |
|||
|
Box 1: Cardiac conditions requiring endocarditis prophylaxis - for high risk procedures |
|||
|
|||
|
Box 2: Recommendations by procedure - for patients with identified cardiac conditions |
|||
|
A. Dental Procedures Antibiotic prophylaxis should only be considered for dental procedures requiring manipulation of gingival or periapical region of teeth or perforation of oral mucosa. Antibiotic prophylaxis is not recommended for local anaesthetic injections in non-infected tissue, removal of sutures, dental X-rays, placement or adjustment of removable prosthodontic or orthodontic appliances or braces, or following shedding of deciduous teeth, or trauma to lips or oral mucosa. |
|||
|
B. Respiratory Tract Procedures Antibiotic prophylaxis should only be considered for invasive procedures involving incision or biopsy of the respiratory mucosa e.g. tonsillectomy or adenoidectomy, or to treat infection e.g. drainage of abscess or empyema. Antibiotic prophylaxis is not recommended for respiratory tract procedures, including bronchoscopy or laryngoscopy, transnasal or endotracheal intubation. |
|||
|
C. Gastrointestinal or genitourinary tract procedures Antibiotic prophylaxis is not recommended for any procedure. |
|||
|
Box 3: Recommended prophylaxis for procedures at risk |
|||
|
Give as a single dose 30 to 60 minutes before procedure |
|||
|
Procedure |
1st line antibiotic |
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
|
See penicillin hypersensitivity section for further information |
|||
|
Dental |
Amoxicillin PO/IV 2g (can give 3g sachet) |
Doxycycline PO 100mg |
Doxycycline PO 100mg |
|
If unable to take oral medication: |
|||
|
CefTRIAXone IV 2g |
Clindamycin IV 600mg |
||
|
Respiratory |
As for dental |
||
Refs:
-
Walter et al. Prevention of Viridans Group Streptococcal Infective Endocarditis. A Scientific Statement from the American Heart Association. Circulation. 2021;143:e963–e978
-
Delgado et al. ESC Guidelines for the management of endocarditis: Developed by the task force on the management of endocarditis of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). European Heart Journal , 2023 ; 44: 3948–4042
Central Nervous System
Suspected Bacterial Meningitis
Suspected Bacterial Meningitis
- The most important aspect of treatment of suspected or confirmed bacterial meningitis is to commence antibacterial therapy IMMEDIATELY .
- Consult with Microbiology or Infectious Diseases is recommended.
- See footnote on use of Dexamethasone** .
- Consult with Microbiology or Infectious Diseases essential if risk factors for M. tuberculosis (alcohol, homelessness, immunocompromised host, recent immigration from area of high incidence, recent contact with tuberculosis) or if history of neurosurgery or head trauma or if device-related infection e.g central nervous system shunt, ventricular drain or other.
- Risk factors for Listeria monocytogenes meningitis in adults include underlying neoplasm, immunosuppressive treatment, age over 50, pregnancy and excessive alcohol use.
- Viral meningitis (as distinct from encephalitis) generally does NOT require anti-viral treatment. Discuss with Microbiology or Infectious Diseases.
- See Appendix 3 for management of contacts.
|
Empiric Antibiotics for Suspected Bacterial Meningitis |
||||
|
Infection |
1 st Line Antibiotics |
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
Comment |
|
See penicillin hypersensitivity section for further information |
||||
|
Suspected Bacterial Meningitis |
CefTRIAXone IV 2g every 12 hours
Consider adding Vancomycin IV infusion, dose per GAPP App calculator, if Pneumococcal meningitis is likely/suspected. See footnote* re vancomycin review and monitoring. See footnote ** re Dexamethasone.
Consider adding Amoxicillin IV 2g every 4 hours if at risk for L. monocytogenes (See point 6 above) |
CefTRIAXone IV 2g every 12 hours
Consider adding Vancomycin IV infusion, dose per GAPP App calculator, if Pneumococcal meningitis is likely/suspected. See footnote* re vancomycin review and monitoring. See footnote ** re Dexamethasone.
Consider adding Co-trimoxazole IV 60mg/kg every 12 hours (round dose to nearest multiple of 480mg) if at risk for L. monocytogenes (See point 6 above) |
Chloramphenicol IV 25mg/kg + Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re vancomycin review and monitoring. Give first dose, THEN IMMEDIATELY consult Microbiology or Infectious Diseases regarding further therapy. See footnote ** re Dexamethasone. Consider adding Co-trimoxazole IV 60mg/kg every 12 hours (round dose to nearest multiple of 480mg) if at risk for L. monocytogenes (See point 6 above)
Discuss need for nasopharyngeal eradication for the patient with Microbiology or Infectious Diseases
|
Minimum duration of treatment:
Meningococcal meningitis: 7 days
Haemophilus meningitis: 10 days
Pneumococcal meningitis: 14 days
Listeria meningitis: 21 days
|
|
* Review need for ongoing vancomycin on a daily basis. For advice on monitoring see Vancomycin Dosing & Monitoring section. |
||||
|
**Dexamethasone
|
||||
Refs:
- HPSC Guidelines for the Early Clinical and Public Health Management of Bacterial Meningitis (including meningococcal disease) November 2016
- ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clinical Microbiology and Infection. 2016; 22 (3); S37-S62
- BNF 86 March 2024
- IDSA Guidelines for the Management of Bacterial Meningitis. Clin Infect Dis 2004;39:1267–84
Suspected Herpes Simplex Encephalitis
Suspected Herpes Simplex Encephalitis
- Viral meningitis (as distinct from encephalitis) generally does NOT require anti-viral treatment. Discuss with Microbiology or Infectious Diseases.
- Consult with Microbiology or Infectious Diseases recommended if patient has recent travel history or is immunocompromised.
|
Antivirals for Suspected Herpes Simplex Encephalitis |
||
|
Infection |
1 st Line
|
Comment |
|
Suspected Herpes Simplex Encephalitis
|
Aciclovir IV 10mg/kg every 8 hours
Refer to IV guide on MedinfoGalway for dosing in obese patients.
|
Confirmed HSV encephalitis requires a total of 14 to 21 days IV therapy. |
Ref: IDSA Guidelines for the Management of Encephalitis Clin Infect Dis 2008;47:303-27
Acute Bacterial Conjunctivitis
Acute Bacterial Conjunctivitis
-
Acute Conjunctivitis can be divided into bacterial, viral, allergic or non-specific aetiologies. It can be difficult to distinguish between them based on clinical exam alone, and all are self-limiting and often resolve within 5–7 days without treatment. Where treatment is required, it is reasonable to manage all as presumed bacterial.
-
This guidance is specifically for acute non gonococcal, non chlamydia bacterial conjunctivitis.
-
If concerned regarding chlamydia or gonococcal conjunctivitis discuss with Microbiology or Infectious Disease AND Ophthalmology.
-
A red eye is generally a sign of inflammation of the conjunctiva (conjunctivitis). Although conjunctivitis is the most common cause of a red eye there are other benign (dry eye, blepharitis, subconjunctival haemorrhage, episcleritis) and sight threatening (uveitis, scleritis, endophthalmitis, acute glaucoma) causes.
-
Conjunctivitis is usually benign so can be managed appropriately by general medical physicians in the first instance so long as certain red flag features (see below) are ruled out.
-
Any of the below red flag features should trigger a same day referral to Ophthalmology. Referral to an ophthalmologist is indicated when the red eye is associated with :
-
Acute reduction in vision of the affected eye
-
Severe pain/photophobia
-
Contact lens use
-
Recent intraocular surgery
-
Recent intravitreal injection
-
Prior glaucoma filtration surgery (Trabeculectomy)
-
Abnormal pupil shape, unequal pupil size or a pupil that reacts poorly to light
-
Copious mucopurulent discharge
-
-
Bacterial conjunctivitis may be associated with mucopurulent discharge and the lids are often “glued” on waking. Send a swab for culture and sensitivity. Topical Chloramphenicol or Fusidic acid may be used.
-
Note Chloramphenicol is not recommended in pregnancy or breastfeeding.
-
Prolonged or recurrent use of any topical antimicrobial agent can lead to the emergence of resistance and should be avoided.
|
Empiric Treatment of Acute Bacterial Conjunctivitis |
||
|
Infection |
Treatment
|
Comment |
|
Acute Bacterial Conjunctivitis |
Chloramphenicol 0.5% drops — apply 2 drops 3 hourly during waking hours or more frequently if required.
Or
Chloramphenicol 1% ointment (Unlicensed) — apply every 6 hours or apply at night only if used in conjunction with chloramphenicol drops.
Or
Fusidic acid 1% eye drops — can be used second line. Apply 1 drop every 12 hours.
|
Duration 48 hours after resolution of symptoms
Note risk of local hypersensitivity reaction to antimicrobial. |
Refs:
- HSE Conjunctivitis - Antibiotic Prescribing Reviewed October 2022
- Summary of Product Characteristics. Chloromycetin 0.5% w/v Redidrops Eye Drops, Solution. Last updated August 2022.
Orbital and Periorbital Cellulitis
Orbital and Periorbital Cellulitis
See Orbital and Periorbital Cellulitis in Skin & Soft Tissue Infections Section
Fungal
Fungal
- Medical assessment is required before prescribing antifungal therapy.
- For suspected oral candidiasis send a swab for fungal culture to microbiology to confirm fungal infection.
- For recurrent or refractory or severe infection send a repeat swab for fungal culture and sensitivities and discuss with Microbiology or Infectious Diseases.
- In immunocompromised patients a high index of suspicion of infection is advised.
|
Empiric Treatment of Fungal Infections |
||
|
Infection |
Treatment |
Comment |
|
Oropharyngeal candidiasis |
Mild Nystatin suspension PO 5ml every 6 hours after food. Swish and swallow, leaving in contact with mouth for at least 30 seconds. |
Duration usually for 7 to 14 days |
|
Moderate to severe Fluconazole PO 100 - 200mg every 24 hours |
Duration usually for 7 to 14 days |
|
|
Fluconazole refractory Contact Microbiology or Infectious Diseases |
||
|
Denture related As above plus disinfection of dentures |
||
|
Oesophageal candidiasis |
Fluconazole PO 200 - 400mg every 24 hours |
Duration 14 to 21 days |
|
Acute Vulvovaginal candidiasis (VVC) |
Recommended regimen (Non-pregnant / non-breastfeeding): Fluconazole capsule 150 mg as a single dose, orally
Recommended topical regimen (if oral therapy contraindicated): Clotrimazole pessary 500 mg as a single dose, intravaginally
Acute VVC in Pregnancy and breastfeeding: Clotrimazole pessary 500 mg intravaginally at night for up to 7 consecutive nights |
Fluconazole refractory/ severe/ recurrent VVC Contact Microbiology or Infectious Diseases |
|
Candida at urinary, respiratory & other sites |
Treatment not routinely indicated. Contact Microbiology or Infectious Diseases |
|
|
Disseminated candidiasis |
Contact Microbiology or Infectious Diseases |
Choice of antifungal depends on sensitivities. |
|
Fungal skin infection |
Contact Microbiology or Infectious Diseases or Dermatology for advice |
|
|
Fungal nail infection |
Contact Microbiology or Infectious Diseases or Dermatology for advice |
|
|
For all other suspected fungal infections e.g. aspergillosis contact Microbiology or Infectious Diseases |
Contact Microbiology or Infectious Diseases for advice |
|
Refs:
- IDSA Candidiasis Guidelines Clin Infect Dis 2016;62:e1-e50
-
British Association for Sexual Health and HIV (BASHH). British Association for Sexual Health and HIV national guideline for the management of vulvovaginal candidiasis (2019). BASHH, 2019.
- HSE Fungal Infections . Antibioticprescribing.ie. November 2023
Gastrointestinal System
Clostridioides difficile Infection (CDI)
Clostridioides difficile Infection (CDI)
- Clinical suspicion of CDI: Diarrhoea (≥3 episodes unformed stool within 24 hours) where patient has been off laxatives for past 24-48 hours
- Detection of C. difficile toxin +/- gene alone does not diagnose CDI. Clinical assessment is essential. Asymptomatic colonisation can occur in 20-40% of hospitalised patients and does not require treatment.
- If CDI diagnosed: See table below for treatment of initial episode and first recurrence of CDI.
- The following regimens may be recommended by Microbiology or Infectious Diseases only
- Regimen for tapered pulsed oral Vancomycin
- Regimen for intracolonic Vancomycin
- Reserve agent recommendation.
|
Antibiotics for Clostridium difficile Infection |
||
|
Infection |
1st Line Antibiotics |
Comment |
|
Clostridioides difficile infection |
Mild: Mildly symptomatic patient ( With NO features of severe CDI ) Metronidazole PO/NG 400mg every 8 hours
IF no response 72 hours after starting treatment, consult Microbiology or Infectious Diseases. |
Duration 10 days.
|
|
All other patients: Vancomycin PO/NG 125mg every 6 hours.
IF severe discuss with Micro/ID
Severe CDI : Suggested by any of the following: Clinical: fever, rigors, abdominal pain Laboratory: WCC ≥15 X 10 9 /L, or rise in serum creatinine >50% above baseline Endoscopic findings: pseudomembranous colitis Imaging: distension of the large intestine, pericolonic fat stranding or colonic wall thickening (including low-attenuation mural thickening). |
||
|
Severe with ileus or toxic megacolon Vancomycin PO/NG 500mg every 6 hours + Metronidazole IV 500mg every 8 hours Consult Microbiology or Infectious Diseases. |
||
|
Clostridioides difficile First or subsequent recurrence or persistent symptoms or patients who are post-Faecal Microbiota Transplant (FMT) for CDI |
Consult Microbiology or Infectious Diseases. |
|
Refs:
- HSE AMRIC National Clostridioides difficile infection (CDI) treatment guidance 2023 https://www.hse.ie/eng/services/list/2/gp/antibiotic-prescribing/conditions-and-treatments/gastro/clostridium-difficile/
- NICE Clostridioides difficile infection: antimicrobial prescribing 2023.
- Clinical Practice Guidelines for the Management of Clostridioides difficile Infection in Adults: 2021 Update by SHEA/IDSA
- European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect 2021 Dec:27 Suppl 2:S1-S21. doi: 10.1016/j.cmi.2021.09.038. Epub 2021 Oct 20.
- Impact of Clostridioides difficile length of treatment on rates of recurrence in patients on concurrent antibiotics Am J Infect Control. 2023 Apr 25:S0196-6553(23)00336.
- Japanese Clinical Practice Guidelines for Management of Clostridioides (Clostridium) difficile infection. Journal of Infection and Chemotherapy. 2022. 28(1045-1083).
- Australasian Society of Infectious Diseases updated guidelines for the management of Clostridium difficile i nfection in adults and children in Australia and New Zealand Intern Med J 2016 Apr;46(4):479-93. doi: 10.1111/imj.13027.
- Clinical Efficacy of Fidaxomicin and Oral Metronidazole for Treating Clostridioides difficile Infection and the Associated Recurrence Rate: A Retrospective Cohort Study Antibiotics 2023, 12, 1323. https://doi.org/10.3390/antibiotics12081323
- Comparison of outcomes with vancomycin or metronidazole for mild‐to‐moderate Clostridium difficile associated diarrhea among solid organ transplant recipients: A retrospective cohort study Transpl Infect Dis 2018 Jun;20(3):e12867. doi: 10.1111/tid.12867.
- Outcomes associated with recent guideline recommendations removing metronidazole for treatment of non-severe Clostridioides difficile infection: a retrospective, observational, nationwide cohort study. CA Gentry, DL Campbell, RJ Williams. Int J Antimicrob Agents 2021 Mar; 57(3):106282. doi: 10.1016/j.ijantimicag.2021.106282. Epub 2021 Jan 17
Tapered pulsed oral Vancomycin
Tapered Pulsed Oral Vancomycin
- Requires Microbiology or Infectious Diseases approval
- Use vancomycin injection to prepare oral solution for inpatients – see IV Guide
- Prescribe vancomycin capsules on discharge. Note: Expensive – not routinely stocked in community. Please contact ward and community pharmacy at least 24hrs prior to discharge to arrange supply.
Vancomycin
- 125mg every 6 hours for 1 week, then
- 125mg every 12 hours for 1 week, then
- 125mg once daily for 1 week, then
- 125mg every second day for 1 week, then
- 125mg every three days for 2 weeks.
Intracolonic Vancomycin
Intracolonic Vancomycin
- Requires Microbiology or Infectious Diseases approval
- Adapted from University of Washington
- Adjunctive therapy for failing Vancomycin therapy in severe CDI
- 500mg of Vancomycin injection is reconstituted and added to 100ml of NaCl 0.9%
- An 18G Foley catheter is inserted per rectum and the balloon is inflated
- The Vancomycin solution is instilled into the rectum and retained for 60 minutes by clamping the catheter
- Once retention time complete, the catheter is unclamped, the balloon deflated and the catheter removed
- The process is repeated every 6 hours
Gastroenteritis
Gastroenteritis
-
Avoid antimicrobial agents unless there is clinical evidence of invasive disease.
-
Consider viral causes if vomiting is a prominent symptom or if norovirus is active in the community or hospital.
-
Maintain hydration.
-
Avoid anti-diarrhoeal agents.
-
Send stool sample (include travel history on the form if relevant).
-
Antimicrobial treatment for gastroenteritis is generally pathogen directed.
-
If there is gastroenteritis with clinical evidence of invasive disease, sepsis, colitis or a history of recent foreign travel or for men who have sex with men (MSM), discuss empiric therapy/management with Microbiology or Infectious Diseases to guide empiric antimicrobial therapy.
Refs:
- IDSA 2017 Treatment guidelines for infectious diarrhoea: Clin Infect Dis 65:1963, 2017.
- Recommendation on aspects of management of shigellosis in Ireland in the context of current antimicrobial resistant Shigella species associated with gay, bisexual and men who have sex with men (gbMSM). HSE; June 2023. https://www.hpsc.ie/a-z/gastroenteric/shigellosis/guidancepublications/Recommendation%20on%20aspects%20of%20management%20of%20shigellosis%20in%20Ireland.pdf
Helicobacter pylori Infection
Helicobacter pylori Infection
- Seek advice from gastroenterologist if 1 st or 2 nd line eradication unsuccessful.
- While choosing a treatment regimen for H. pylori , patients should be asked about previous antibiotic exposure and this information should be incorporated into the decision-making process.
- Please consider medication side effects and interactions, when choosing a triple therapy regime.
- Second-line therapy depends on the first-line therapy and should not be the same treatment.
- Following triple therapy, there is no need to continue acid- inhibiting treatments (PPI). However, if the ulcer is large, duodenal or complicated by haemorrhage or perforation, acid-inhibiting treatments can be continued for a longer duration. Patients should be maintained on the lowest effective dose of acid inhibiting treatment on an ‘as required’ basis.
- Testing for eradication is recommended in all patients treated for H. pylori and should occur at least 6 - 8 weeks following treatment. Please inform the patient and the GP - a H. pylori stool antigen test should be performed 6 - 8 weeks after H. pylori eradication. To increase accuracy, patients must not be on any medication that affects H. pylori detection; these include antibiotics (past 4 weeks), PPIs (past 2 weeks), and bismuth (past 4 weeks). If symptomatic relief is required during this period, H2 receptor antagonists and anti-acid medications are recommended.
- Referral for OGD for H. pylori culture and susceptibility testing should be performed following two treatment regime failures.
- Bismuth is available in Ireland as unlicensed medicines (ULM) – and therefore not routinely stocked in community. Please contact ward and community pharmacy at least 24hrs prior to discharge to arrange supply.
- Newer generation PPIs, e.g. esomeprazole 40mg, are considered more effective than first generation PPIs.
|
Antibiotic regimens for Helicobacter pylori Eradication |
||||
|
1 st Line Helicobacter pylori eradication
|
1 st Line Therapy |
Alternative 1 st Line Therapy
OR
|
Comment |
|
|
Esomeprazole PO 40mg (PPI) every 12 hours + Clarithromycin PO 500mg every 12 hours + Amoxicillin 1g PO every 12 hours |
Esomeprazole PO 40mg (PPI) every 12 hours + Bismuth subcitrate PO 120mg every 6 hours (ULM) + Metronidazole PO 400mg every 8 hours + Doxycycline PO 100mg every 12 hours
|
Duration: 14 days
|
||
|
2 nd line Helicobacter pylori eradication - if still infected after 1 st line therapy
|
2 nd Line Therapy |
Alternative 2 nd Line Therapy
|
|
|
|
NO Penicillin Allergy |
Penicillin Allergy |
|
||
|
|
||||
|
Esomeprazole PO 40mg (PPI) every 12 hours + Clarithromycin PO 500mg every 12 hours + Metronidazole PO 400mg every 12 hours
|
Esomeprazole PO 40mg (PPI) every 12 hours + Levofloxacin PO 250mg every 12hours + Amoxicillin PO 1g every 12hours |
Esomeprazole PO 40mg (PPI) every 12 hours + Bismuth subcitrate PO 120mg every 6 hours (ULM) + Metronidazole PO 400mg every 8 hours + Doxycycline PO 100mg every 12 hours
|
|
|
Refs:
- HSE Helicobacter pylori – Antibiotic Prescribing.ie November 2023
- Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Malfertheiner P, et al. Gut 2022;71:1724–1762. doi:10.1136/gutjnl-2022-327745 Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report (bmj.com)
Genital System
Genital System
Discuss with Infectious Diseases or Microbiology
|
Empiric Antibiotics for Genital System Infections |
|||
|
Infection |
1 st Line Antibiotics |
Penicillin allergy: immediate or severe delayed reaction |
Comment |
|
See penicillin hypersensitivity section for further information |
|||
|
Pelvic Inflammatory Disease - Outpatient Treatment Send a full STI screen including Chlamydia & Gonorrhoea |
CefTRIAXone IM 1g single dose + Doxycycline PO 100mg every 12 hours + Metronidazole PO 400mg every 12 hours |
Ofloxacin PO 400mg every 12 hours (see Fluoroquinolone warning ) + Metronidazole PO 400mg every 12 hours |
Duration 14 days |
|
Pelvic Inflammatory Disease - Inpatient Treatment Send a full STI screen including Chlamydia & Gonorrhoea |
CefTRIAXone IV 2g every 24 hours + Doxycycline PO 100mg every 12 hours + Metronidazole PO 400mg every 12 hours |
Clindamycin IV 900mg every 8 hours + Gentamicin IV every 24 hours, dose per GAPP App calculator. See footnote* re review and monitoring. Followed by Doxycycline PO 100mg every 12 hours + Metronidazole PO 400mg every 12 hours Note: The initial IV regimen does not cover Chlamydia or Gonorrhoea. It is important to send a full STI screen. |
Stop IV treatment 24 hours after clinical improvement
Continue oral antibiotic therapy for a total duration of 14 days to complete the course |
|
Acute Prostatitis/ Epididymo-orchitis If Sexually active |
CefTRIAXone IM 1g single dose (or 2g IV if inpatient) + Doxycycline PO 100mg every 12 hours Send urethral swabs for Chlamydia & Gonorrhoea if sexually active in past six months & refer to Sexually Transmitted Infection (STI) Clinic/Infectious Diseases. |
Duration oral doxycycline of 14 days.
Consider mumps as aetiology. |
|
|
Acute Prostatitis/ Epididymo-orchitis If NOT sexually active |
Ciprofloxacin PO 500mg every 12 hours (See Fluoroquinolone warning ) IF patient appears septic treat as suspected bloodstream infection: Add Gentamicin IV one dose per GAPP App calculator. See footnote* re further doses and monitoring. |
Duration 14 to 28 days |
|
|
* Review need for ongoing Gentamicin on a daily basis. Continue with once daily Gentamicin dosing ONLY if Consultant/Specialist Registrar recommended. For advice on monitoring see Aminoglycoside Dosing & Monitoring section. |
|||
Refs:
- BASHH UK National Guideline for the Management of Pelvic Inflammatory Disease (2019 Interim Update)
- BASHH UK National Guideline for the Management of Epididymo-orchitis 2019
- Prostatitis (acute): antimicrobial prescribing NICE guideline Published: 31 October 2018
Intravascular Line
Intravascular Line
- Blood cultures should be taken if the patient appears septic and/or if the patient has a central or peripheral vascular catheter (CVC/PVC) exit site infection indicated by the presence of cellulitis or thrombophlebitis. One set of blood cultures from a peripheral vein and one set from all lumens of central line should be sent at the same time with each site clearly labelled on the form.
- If evidence of exit site infection , a swab should be taken from the site and the line should be removed.
- In the setting of suspected central line infection, the tip of the central line should be sent to the microbiology lab in a universal container for culture and sensitivity testing, cut to 4 cm in length following removal.
- If blood cultures are positive discuss with Microbiology or Infectious Diseases.
-
Infection at the site of CVC/PVC, with no systemic features of sepsis and with negative blood cultures may be treated as a skin/soft tissue infection. Treatment is as follows:
- Removal of the catheter is essential
- Initial therapy should be with Vancomycin IV
- Review at 48 hours, and consider switch to Flucloxacillin or other antibiotic if appropriate, based on culture and sensitivity results and MRSA screens.
- An antibiotic lock solution is very occasionally recommended by Microbiology or Infectious Diseases. If indicated, contact pharmacy for protocol.
|
Empiric Antibiotics for Intravascular Line Infections |
||
|
Infection |
1 st Line |
Comment |
|
Central and Peripheral IV Catheter Exit Site Infection See notes above |
Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re monitoring. Review at 48 hours, change to pathogen directed therapy based on culture & sensitivity |
Duration 7 to 10 days |
|
Peripheral Line-related Infection and/or Bacteraemia |
Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re monitoring. Review at 48 hours, change to pathogen directed therapy based on culture & sensitivity |
Duration minimum 14 days, for exceptions see algorithm for NON-tunnelled CVC bacteraemia |
|
Central Line-related Infection and/or Bacteraemia Remove CVC and send tip to microbiology |
Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re monitoring. + Gentamicin IV one dose per GAPP App calculator and review . See footnote* re further doses and monitoring. If blood cultures are positive treat as per Microbiology or Infectious Diseases advice. |
Duration varies by type of line, organism and complications. A prolonged course may be required. See algorithms for NON-tunnelled CVC bacteraemia and tunnelled CVC/port bacteraemia When denoting duration of antimicrobial therapy day 1 is the first day on which negative blood cultures are obtained. |
|
* Review need for ongoing Gentamicin & Vancomycin on a daily basis. Continue with once daily Gentamicin dosing ONLY if Consultant/Specialist Registrar recommended. For advice on monitoring see Aminoglycoside & Vancomycin Dosing & Monitoring section. |
||
Refs:
- HSE/RCPI/HCAI Prevention of Intravascular Catheter-related Infection in Ireland Partial Update of 2009 Guidelines September 2014
- IDSA Guidelines for the diagnosis and management of intravascular catheter-related infection. Clin Infect Dis 2009;49:1-45
Algorithm for Management of NON-tunnelled CVC Bacteraemia
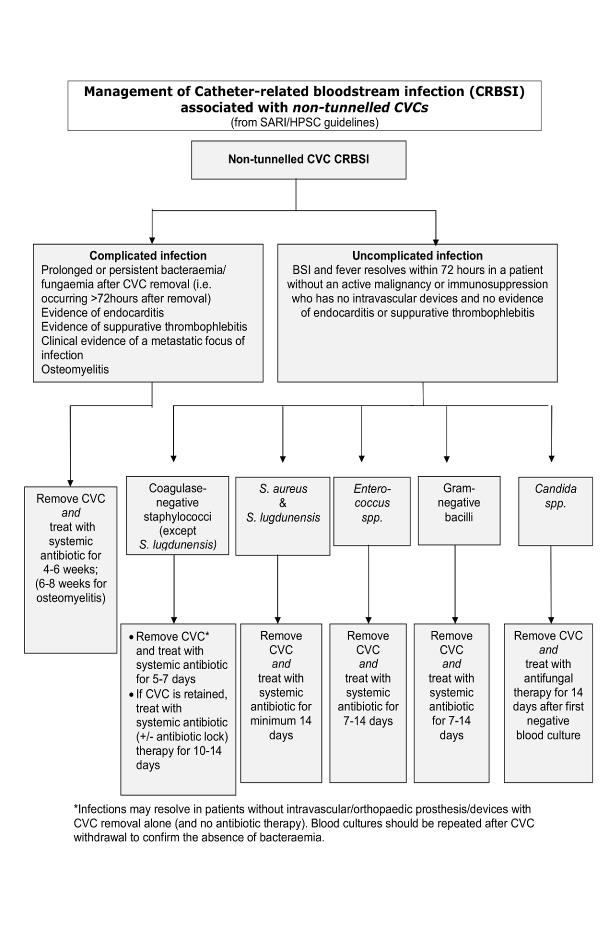
(click on image to enlarge)
Algorithm for Management of Tunnelled CVC / Port Bacteraemia
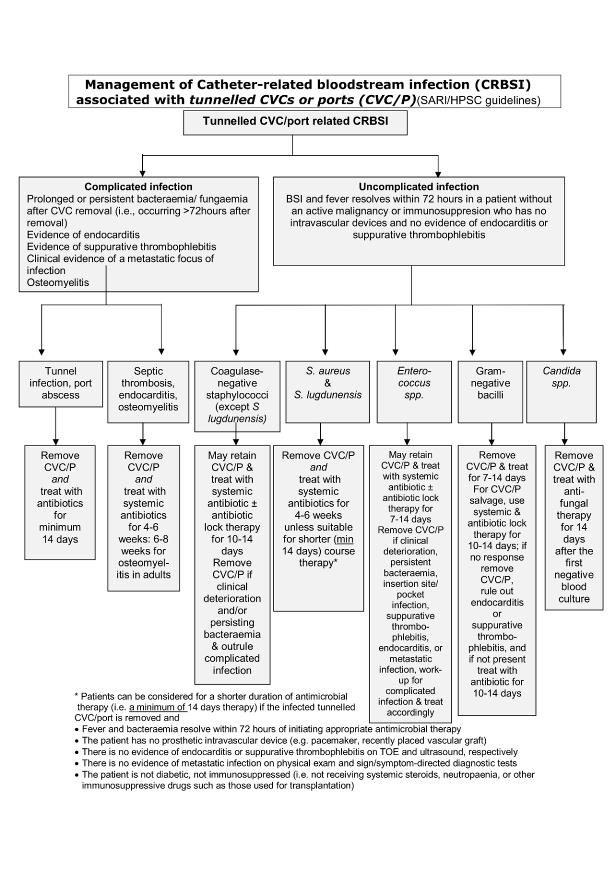
(click on image to enlarge)
Malaria
Malaria
-
Discussion with Infectious Diseases or Microbiology recommended.
-
Species of infecting parasite is frequently uncertain .
-
Severe malaria is a medical emergency. After rapid clinical assessment, a diagnostic test should be sent. In patients with clinically severe malaria or high parasitaemia (2% or greater) first line treatment is intravenous Artesunate which should be started within one hour of assessment. Intravenous Quinine (Unlicensed) may be used if Artesunate is unavailable for any reason.
-
Follow-on therapy Note for some kinds of malaria additional follow on therapy with Primaquine is required to eradicate the persistent liver stage. All cases must be discussed with Infectious Diseases or Microbiology.
-
Discharge prescriptions There are frequent problems with availability and medical card coverage of oral treatment on discharge. Please contact ward and community pharmacy at least 24hrs prior to discharge to arrange supply.
|
Malaria Treatment |
||||
|
Indication |
Oral Antimalarials |
Comment
|
||
|
1 st Line |
2 nd & 3 rd line |
|||
|
Non-severe malaria Non-Pregnant Adult
|
Riamet® PO (over 35kg body weight) 4 tablets at 0,8,24,36,48,60 hours (total 24 tablets over 60 hours)
(Four tablets of Riamet® contain 80mg of Artemether & 480mg of Lumefantrine) |
Second line: Malarone® PO 4 tablets every 24 hours for 3 days (Four tablets of Malarone® contain 1g of Atovaquone & 400mg of Proguanil)
OR
Third line option - non-pregnant adult only: Quinine PO 600mg (Unlicensed) every 8 hours + Doxycycline PO 200mg every 24 hours
Duration 7 days
|
Avoid quinine if hypersensitive |
|
|
Non-severe malaria Pregnant Adult |
All Trimesters : Riamet® PO (over 35kg body weight) 4 tablets at 0,8,24,36,48,60 hours (total 24 tablets over 60 hours)
(Four tablets of Riamet® contain 80mg of Artemether & 480mg of Lumefantrine) |
Second line: Quinine PO 600mg every 8 hours + Clindamycin PO 450mg every 8 hours
Duration 7 days
|
|
|
|
Severe Malaria Treat as a medical emergency. See notes above. |
Intravenous Antimalarials: If seriously ill or unable to take tablets |
Comment
|
||
|
1 st Line |
2 nd line (if artesunate not available). Avoid quinine if hypersensitive. |
|||
|
Artesunate IV 2.4mg/kg at 0, 12, 24 hours, then every 24 hours until oral treatment can be substituted (see below).
|
Quinine IV infusion (Unlicensed) Loading dose* : 20mg/kg (maximum 1.4g) infused over 4 hours, followed 8 hours after start of the loading dose by Maintenance dose : 10mg/kg (maximum 700mg) IV Infusion (over 4 hours) every 8 hours Reduce maintenance dose to 5 to 7 mg/kg (maximum 700mg) every 8 hours in severe renal impairment, severe hepatic impairment, or if IV treatment continues for more than 48 hours. *Do NOT give loading dose if patient has received quinine or mefloquine in previous 12 hours
Oral switch after 24 hours: see below. |
Give intravenous antimalarials in the treatment of severe malaria for a minimum of 24 hours -irrespective of the patient’s ability to tolerate oral medication earlier. Quinine toxicity: ECG monitoring required in the elderly and patients with cardiac disease Significant risk of hypoglycaemia with IV quinine. Monitor blood glucose regularly (about every 2 hours) in the acute situation. |
||
|
A full course of oral treatment should always follow IV artesunate (see below). This applies even if the duration of IV treatment was for 2 days or more. When the patient has had at least 24 hours of IV artesunate (doses at 0, 12, 24 hours) and the patient is able to swallow and absorb medication, give Riamet ® PO, starting at least 4 hours after the final dose of IV artesunate. Riamet® PO (over 35kg body weight) 4 tablets at 0,8,24,36,48,60 hours (total 24 tablets over 60 hours) (Four tablets of Riamet® contain 80mg of Artemether & 480mg of Lumefantrine) |
Switch to oral therapy after the first 24 hours (3 doses) to complete a full oral course when the patient is able to swallow AND retain oral medication by giving a course of: Riamet® PO (over 35kg body weight) 4 tablets at 0,8,24,36,48,60 hours (total 24 tablets over 60 hours) (Four tablets of Riamet® contain 80mg of Artemether & 480mg of Lumefantrine) |
|||
- WHO Guidelines for Malaria October 2023
- Lalloo et al UK malaria treatment guidelines 2016 Journal of Infection 72:635-649
- The Sanford Guide to Antimicrobial Therapy Digital update March 2024
Neutropenic Sepsis
Neutropenic Sepsis
- Any suspicion of neutropenia and fever OR clinical signs of sepsis must be assessed immediately as an emergency
- Fever means temperature ≥38.3ºC on one occasion or sustained temperature greater than 38ºC.
- Neutropenia means an absolute neutrophil count of less than 0.5 X 10 9 /L.
- Administer antimicrobials promptly once sepsis is suspected. HSE Sepsis Programme Documents & Resources are available at https://www.hse.ie/eng/about/who/cspd/ncps/sepsis/resources/
- Note frequent review is essential. The time frames suggested for addition of additional empiric therapy may need to be shortened if the patient’s condition is deteriorating.
- Consider risk for fungal infection and viral infection.
- If the infection is CVC associated - remove the CVC .
- Review previous microbiology for history of colonisation or infection with antibiotic resistant organisms and assess other risk factors for antibiotic resistance. If colonised with Multi-drug Resistant Organisms (MDRO) including Carbapenemase Producing Enterobacteriacae (CPE), discuss with Microbiology or Infectious Diseases.
- Comprehensive Haematology Guidelines are available on QPulse.
- Summary treatment algorithms:
Refs:
- IDSA Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2011;52:e56-93
- GUH Haematology Guidelines for the management of febrile neutropenic patients (QPulse CLN-HAEM-020)
- NICE Neutropenic Sepsis: prevention and management in people with cancer ( Clinical guideline 151 ) 2012
- Adult Sepsis Form 2021
Initial management of neutropenic sepsis - Algorithm
(click on image to enlarge)
Continuing management of neutropenic sepsis - Algorithm
(click on image to enlarge)
Obstetrics
Obstetrics
- These are summary empiric antibiotic choices. For full detailed guidance see Women’s and Children’s (WAC) Directorate Guidelines on QPulse. See listed references below.
- Discussion with Microbiology or Infectious Diseases recommended for patients showing signs of sepsis.
- Identify need for further intervention to address the source of infection e.g. drainage or removal of source.
- Consider country of origin and travel history, particularly travel in areas with risk for transmission of malaria, dengue fever or TB.
- The regimens below may NOT cover Multi-drug Resistant Organisms (MDRO) in all cases. See note on MDRO .
- Duration of treatment & oral switch is decided on a case-by-case basis depending on subsequent diagnosis as well as clinical progress.
|
Empiric Antibiotics for Obstetric Infections |
||||
|
Infection |
1 st Line Antibiotics |
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
Comment |
|
See penicillin hypersensitivity section for further information |
||||
|
The regimens below may NOT cover Multi-drug Resistant Organisms (MDRO) in all cases. See note on MDRO |
||||
|
Chorioamnionitis Discuss considerations around delivery with consultant obstetrician Or Endometritis (Post-partum) If sepsis follow antibiotic treatment for sepsis For full detailed guidance see WAC Directorate Guideline on the Management of Suspected Sepsis and Sepsis in Obstetric Care (QPulse CLN- OGCP-218) |
Co-amoxiclav IV 1.2g every 8 hours + Gentamicin IV one dose per GAPP App calculator (use booking weight). See footnote* re further doses and monitoring |
CefTRIAXone IV 2g every 24 hours + Gentamicin IV one dose per GAPP App calculator (use booking weight). See footnote* re further doses and monitoring + Metronidazole IV 500mg every 8 hours |
Discuss with Microbiology or Infectious Diseases Ciprofloxacin IV 400mg every 12 hours. See footnote^ re use in pregnancy + Gentamicin IV one dose per GAPP App calculator (use booking weight). See footnote* re further doses and monitoring See footnote^ re use in pregnancy. + Vancomycin IV infusion, dose per GAPP App calculator (use booking weight). See footnote* re monitoring + Metronidazole IV 500mg every 8 hours |
|
|
Intrapartum Antibiotic Prophylaxis (IAP) |
For full detailed guidance see WAC Directorate Guidelines and Procedure for the Management of Group B Streptococus (QPulse CLN-LW-0033) |
|||
|
Preterm Prelabour R upture of Membranes With NO evidence of sepsis/ Chorioamnionitis |
For full detailed guidance see WAC Directorate Preterm Prelabour Rupture of Membranes (PPROM) (QPulse CLN-LW-0012) |
Duration 10 days |
||
|
Erythromycin PO 250mg every 6 hours |
||||
|
Mastitis |
For full detailed guidance see WAC Directorate Guideline on the Management of Mastitis and Breast Abscess in the Lactating Woman (QPulse CLN-OGCP-275) See Skin/Soft Tissue Section for summary empiric treatment options for Cellulitis / Mastitis |
|||
|
Sepsis |
For full detailed guidance, including antibiotics , see WAC Directorate Guideline on the Management of Suspected Sepsis and Sepsis in Obstetric Care (QPulse CLN-OGCP-218) See Sepsis Section for summary empiric treatment options for Sepsis in pregnancy (includes options in penicillin allergy ) |
|||
|
Urinary Tract Infection |
For full detailed guidance see WAC Directorate Management of Urinary Tract Infections in Pregnancy (QPulse CLN-OGCP-227) See Urinary Tract Section for summary empiric treatment options for acute pyelonephritis in pregnancy |
|||
|
^Gentamicin & Ciprofloxacin are recommended in pregnancy when benefit outweighs risk. * Review need for ongoing Gentamicin on a daily basis. Continue with once daily Gentamicin dosing ONLY if Consultant / Specialist Registrar recommended. For advice on monitoring see Aminoglycoside Dosing & Monitoring section. |
||||
Refs:
- WAC Directorate Guideline on the Management of Mastitis and Breast Abscess in the Lactating Woman (QPulse CLN-OGCP-275)
- WAC Directorate Guideline on the Management of Suspected Sepsis and Sepsis in Obstetric Care (QPulse CLN- OGCP-218)
- WAC Directorate Management of Urinary Tract Infections (UTI’s) in Pregnancy (QPulse CLN-OGCP-227)
- WAC Directorate Preterm Prelabour Rupture of Membranes (PPROM) (QPulse CLN-LW-0012)
- WAC Directorate Guideline on the Management of Pyrexia in Labour (QPulse CLN-LW-0034)
- WAC Directorate Guideline and Procedure for the Management of Group B Streptococus (QPulse CLN-LW-0033)
- Royal College of Obstetrics and Gynaecologists (RCOG). Bacterial Sepsis in & following Pregnancy, Green-top Guidelines 64a & 64b 2 012 .
- Royal College of Physicians Ireland (RCPI). Prevention of Early-Onset Group B Streptococcal Disease in Term Infants . National Clinical Practice Guideline. HSE National Women &Infants Health Programme/ Institute of Obstetricians & Gynaecologists, 2023
Respiratory System
Antivirals Guidance for Treatment and Prophylaxis of Influenza
Detailed guidance on the use of antiviral agents for the treatment and prophylaxis of influenza is available on the HSE/HPSC website https://www.hpsc.ie/a-z/respiratory/influenza/seasonalinfluenza/guidance/antiviraltreatmentandprophylaxisguidance/Antivirals%20guidance%20for%20treatment%20and%20prophylaxis%20of%20%20influenza.pdf
Antiviral treatment
- Antiviral treatment is recommended as early as possible for any patient with suspected or confirmed influenza who is hospitalised due to influenza
- Any patient who, while in hospital for other indication, develops influenza, should be assessed for risk from influenza complications (as below). Antiviral treatment is recommended as early as possible for those at higher risk from influenza complications.
Higher risk from influenza complications includes :
- Age 65 years and over
- Pregnancy (including up to two weeks post-partum)
- Children aged <2 years of age
- Chronic respiratory disease including those on medication for asthma
- Chronic heart, kidney, liver or neurological disease
- Diabetes mellitus
- Haemoglobinopathies
- Immunosuppression (whether due to treatment or disease e.g. HIV)
- Morbid obesity (BMI ≥ 40)
- Those with any condition that can compromise respiratory function (e.g cognitive dysfunction, spinal cord injury, seizure disorder, or other neuromuscular disorder), especially those attending special schools/day centres.
- Those with Down Syndrome
- Persons with moderate to severe neurodevelopmental disorders such as cerebral palsy and intellectual disability
- Residents of nursing homes or Residential Care Facilities
- 1 st line antiviral treatment is generally PO/NG Oseltamivir 75mg BD for adult patients with normal renal function but:
- Dose Adjustment is required in renal impairment .
- Alternative therapy with Zanamivir may be indicated for patients with severe immunocompromise – please discuss with Microbiology/Infectious Diseases
- Treatment duration is generally 5 days.
There is limited evidence to support treating for longer duration in those with severe influenza (e.g critically ill in ICU) and in severely immunosuppressed patients. Discussion with Microbiology/Infectious Diseases is recommended.
Antiviral prophylaxis
Chemoprophylaxis (generally PO/NG Oseltamivir) may be considered for people at higher risk from influenza complications (as above, 1 to 13) who have had recent close contact with a person with influenza. Details on use of Oseltamivir for prophylaxis of high risk contacts, including dose, duration and dose adjustment in renal impairment are to be found in the HPSC/ HSE guidance
Community Acquired Pneumonia
Community Acquired Pneumonia (CAP)
- Community acquired pneumonia is defined as infiltrate on CXR or CT scan with compatible symptoms.
- Antibiotics are NOT usually recommended for exacerbation of asthma or bronchitis with normal chest X-ray or aspiration with normal CXR .
- Nursing home patients presenting with pneumonia should be treated as CAP as outlined below and NOT automatically treated with piperacillin/tazobactam unless history of antibiotic resistant organisms or within 14 days of discharge from hospital.
- The CURB-65 score , in conjunction with clinical judgement, is a severity assessment tool for Community Acquired Pneumonia.
- Laboratory testing for respiratory viruses should be considered, including COVID-19, and, during relevant season, influenza and Respiratory Syncytial Virus (RSV). Appropriate treatment for COVID-19 or influenza should be initiated if positive.
-
Culture sputum and blood if severe infection
OR
risk factors for MRSA or Pseudomonas infections:
- ICU admission
- Hospitalised and/or IV antibiotics within past 90 days
- Previous Infection with MRSA or Pseudomonas
- Give antibiotics as soon as possible, within 4 hours of presentation in the Emergency Department.
|
Empiric Antibiotics for Community Acquired Pneumonia (CAP) |
|||||
|
Infection |
1 st Line Antibiotics |
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
Comment |
|
|
See penicillin hypersensitivity section for further information |
|||||
|
Community Acquired Pneumonia (including nursing home patients unless history of MDRO or within 14 days of discharge from hospital). See note on MDRO
Signs and symptoms of LRTI AND new consolidation on chest X-ray |
Mild CURB-65 Score 0 or 1 |
||||
|
Amoxicillin PO 1g every 8 hours
In younger patients Add atypical cover with Clarithromycin PO 500mg every 12 hours |
Doxycycline PO 100mg every 12 hours
Avoid Doxycycline in pregnancy or breast-feeding. Discuss with Microbiology or Infectious Diseases. |
Duration 5 days (provided afebrile and clinically stable for 48 hours. Otherwise 7 days)
|
|||
|
Moderate CURB-65 Score 2 |
|||||
|
Non-smokers with no co-morbidities Amoxicillin PO/IV 1g every 8 hours + Clarithromycin PO (IV if NPO) 500mg every 12 hours
Patients who smoke and/or with co-morbidities
Co-amoxiclav PO 875/125mg every 8 hours/IV 1.2g every 8 hours + Clarithromycin PO (IV if NPO) 500mg every 12 hours |
Levofloxacin PO (IV if NPO) 500mg every 12 hours
Avoid Levofloxacin in pregnancy or breast-feeding. Discuss with Micro/ID. Caution if risks for prolonged QT interval |
Duration 5 days (provided afebrile and clinically stable for 48 hours. Otherwise 7 days )
Most patients can be treated with oral antibiotics |
|||
|
Severe CURB-65 Score ≥ 3 |
|||||
|
Co-amoxiclav IV 1.2g every 8 hours + Clarithromycin PO (IV if NPO) 500mg every 12 hours |
CefTRIAXone IV 2g every 24 hours + Clarithromycin PO (IV if NPO) 500mg every 12 hours |
Levofloxacin PO (IV if NPO) 500mg every 12 hours Avoid levofloxacin in pregnancy or breastfeeding. Discuss with Micro/ID. Caution if risks for prolonged QT interval. |
Duration 7 days Longer courses may be indicated according to clinical judgement e.g. if Legionella pneumophila, Staphylococcus aureus or Gram-negative bacilli suspected or confirmed.
Consider addition of steroids for those requiring Non Invasive Ventilation (NIV)/ Mechanical Ventilation (MV) in consultation with Resp/ID. |
||
Refs:
- Community-Acquired Pneumonia. The New England Journal of Medicine. 2023. 389:632-41.
- Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care 2019;200(7):e45–e67
- Duration of Antibiotic Treatment in Community-Acquired Pneumonia: A Multicenter Randomized Clinical Trial. JAMA Intern Med 2016;176(9):1257-1265
- British Thoracic Society Guidelines for the management of community acquired pneumonia in adults. Annotated CAP Guideline 2015.
Chronic Obstructive Pulmonary Disease (COPD)
|
Empiric Antibiotics for COPD |
|||
|
Infection |
1st Line Antibiotics |
See penicillin hypersensitivity section for further information |
Comment |
|
COPD Exacerbation without infiltrate
|
Amoxicillin PO 1g every 8 hours OR If recent (<2/52) course of amoxicillin: Co-amoxiclav PO 625mg every 8 hours (Consider Co-amoxiclav PO 875/125mg every 8 hours for severe infection) |
Clarithromycin PO 500mg every 12 hours OR Doxycycline PO 100mg every 12 hours Avoid doxycycline in pregnancy or breastfeeding. |
Duration 5 days (provided afebrile and clinically stable for 48 hours. Otherwise 7 days) |
Refs:
Hospital Acquired Pneumonia
Hospital Acquired Pneumonia
- Pneumonia should be treated as hospital acquired if onset from 5 days after hospital admission or within 14 days of discharge.
- Nursing home patients presenting for admission to hospital with pneumonia should be treated as CAP and NOT automatically treated with piperacillin/tazobactam unless history of antibiotic resistant organisms or within 14 days of discharge from hospital.
- The regimens below may NOT cover Multi-drug Resistant Organisms (MDRO) in all cases. See note on MDRO .
- Intensive care and immunosuppressed patients should be discussed with Microbiology or Infectious Diseases.
|
Empiric Antibiotics for Hospital Acquired Pneumonia |
|||||
|
Infection |
1 st Line Antibiotics |
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
Comment
|
|
|
See penicillin hypersensitivity section for further information |
|||||
|
The regimens below may NOT cover Multi-drug Resistant Organisms (MDRO) in all cases. See note on MDRO |
|||||
|
Hospit al Acquired Pneumonia
Contact Micro/ ID for treatment of Pseudomonal infection
|
Moderate Piperacillin/tazobactam IV 4.5g every 6 hours
|
Moderate CefTRIAXone IV 2g q24h NB. If history of Pseudomonas colonisation/infection, discuss alternative treatment with Micro/ID.
Add Gentamicin IV IF sepsis . Give one dose per GAPP App calculator. See footnote* re further doses and monitoring. |
Moderate Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re monitoring. + Ciprofloxacin** IV 400mg every 12 hours
Add Gentamicin IV IF sepsis . Give one dose per GAPP App calculator. See footnote* re further doses and monitoring.
Discuss treatment at 48 hours with Micro/ID |
Duration 7 days
May need to be extended according to clinical judgement e.g. if Legionella pneumophila, Staphylococcus aureus or Gram-negative bacilli suspected or confirmed |
|
|
Severe (ICU assessment required) Piperacillin/tazobactam IV 4.5g every 6 hours + Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re review and monitoring. Review at 24 - 48 hours and stop if MRSA not detected from clinical samples or MRSA screen
Add Gentamicin IV IF sepsis . Give one dose per GAPP App calculator. See footnote* re further doses and monitoring |
Severe (ICU assessment required) Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re monitoring. + Ciprofloxacin** IV 400mg every 12 hours
Add Gentamicin IV IF sepsis . Give one dose per GAPP App calculator. See footnote* re further doses and monitoring.
|
||||
|
|
|||||
|
|
* Review need for ongoing Gentamicin and Vancomycin on a daily basis. Continue with once daily Gentamicin dosing ONLY if C onsultant/Specialist Registrar recommended. For advice on monitoring see Aminoglycoside & Vancomycin Dosing & Monitoring section. **Switch from IV to oral Ciprofloxacin (500mg PO every 12 hours) as soon as possible. |
||||
Refs:
Pneumocystis jirovecii pneumonia (PJP)
Pneumocystis jirovecii pneumonia (PJP)
- Discussion with Microbiology or Infectious Diseases recommended.
- Co-trimoxazole in high dosage is the treatment of choice for mild, moderate and severe PJP.
- For moderate to severe disease (Pa02 ≤9kPa on room air), high dose steroids should be co-administered with anti-pneumocystis therapy and should be discontinued before anti-pneumocystis therapy is complete: Prednisolone 40mg PO twice daily for 5 days, then 40mg once daily for 5 days, then 20mg once daily to complete a total of 14 to 21 days (depending on duration of PJP treatment).
|
PJP Treatment |
|||
|
Infection |
1 st Line |
2 nd line
|
Comment |
|
PJP
|
Co-trimoxazole* IV/PO 120mg/kg/ day divided into a 6 to 8 hourly dosing regimen e.g. 30mg/kg every 6 hours*
e.g. 70kg patient: 70x120 = 8,400mg daily, dosing regimen would be 2,100mg every 6 hours (round dose to nearest 480mg=1920mg)
In severe disease consider oral switch at same dose when clinically improving. In mild to moderate disease consider oral route from outset.
|
Severe disease: Pentamidine IV 4mg/kg once daily Only to be used if intolerant or unresponsive to co- trimoxazole. Risk of significant adverse events including severe hypotension and hypoglycaemia with administration.
Non-severe disease: Atovaquone OR Dapsone + Trimethoprim OR Clindamycin + Primaquine
Contact Microbiology or Infectious Diseases or Pharmacy for advice and dosing.
|
Duration (ID or Micro consult recommended): Non-HIV infected: 14 -21 days HIV infected: 21 days
|
|
*Please note the co-trimoxazole dose is a combined trimethoprim/sulfamethoxazole dose Caution with dose calculation as errors have occurred when dosing is based on the trimethoprim component (as recommended in US literature) |
|||
Refs:
- Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Available at https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection. Accessed 23rd April 2024.
- Pneumocystis jirovecii pneumonia. Catherinot et al. Infect Dis Clin North Am 2009;24:107-138
Sepsis - Source Unclear
Sepsis - Source Unclear
- Discussion with Microbiology or Infectious Diseases recommended .
- If source is known or suspected e.g. meningitis, respiratory, urinary, skin and soft tissue, ensure antibiotics are appropriate for the source. Follow the antibiotic recommendations in the corresponding chapter.
- Identify need for further intervention to address the source of infection e.g. drainage or removal of source.
- The regimens below may NOT cover Multi-drug Resistant Organisms (MDRO) in all cases. See note on MDRO .
- Administer antimicrobials promptly once sepsis is suspected. HSE Sepsis Programme Documents & Resources (including Screening form and algorithm) are available at https://www.hse.ie/eng/about/who/cspd/ncps/sepsis/resources/
- If infection site is known, culture results are available, and/or patient improved, review treatment with new information and consider de-escalation. If antibiotics are still required, use the narrowest spectrum of coverage for the shortest time.
- Duration of treatment is decided on a case-by-case basis depending on subsequent diagnosis as well as clinical progress.
|
Empiric Antibiotics for Sepsis – Source Unclear |
|||
|
Infection |
1 st Line Antibiotics
|
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
|
See penicillin hypersensitivity section for further information |
|||
|
The regimens below may NOT cover Multi-drug Resistant Organisms (MDRO) in all cases. See note on MDRO |
|||
|
Antibiotics must be given as soon as possible, then discuss with Microbiology or Infectious Diseases. Meropenem should be considered in patients who are critically ill with sepsis or have a history of a Gram-negative Multi-drug Resistant Organism (MDRO). Discuss use of Meropenem with Microbiology or Infectious Diseases. If meropenem is essential in a patient with a history of severe penicillin allergy e.g. anaphylaxis, close monitoring is required for cross sensitivity e.g. in ICU. |
|||
|
Sepsis – Source Unclear
No risk factors for MRSA e.g. No CVC/ No IV Drug Use |
Give antibiotics immediately |
||
|
Piperacillin/tazobactam IV 4.5g every 6 hours + Gentamicin IV one dose per GAPP App calculator. See footnote 1 re further doses and monitoring. See footnote 2 re use in pregnancy.
See footnote 3 re sepsis in pregnancy.
|
CefTRIAXone IV 2g every 24 hours +
Gentamicin IV one dose per GAPP App calculator. See footnote 1 re further doses and monitoring. See footnote 2 re use in pregnancy.
If pregnant or suspected intra-abdominal source: Add Metronidazole IV 500mg every 8 hours
See footnote 3 re sepsis in pregnancy. |
Discuss with Microbiology or Infectious Diseases Ciprofloxacin IV 400mg every 12 hours. See footnote 2 re use in pregnancy. + Gentamicin IV one dose per GAPP App calculator. See footnote 1 re further doses and monitoring. See footnote 2 re use in pregnancy. + Vancomycin IV infusion, dose per GAPP App calculator. See footnote 1 re monitoring.
If pregnant or suspected intra-abdominal source: Add Metronidazole IV 500mg every 8 hours
See footnote 3 re sepsis in pregnancy.
|
|
|
Sepsis – Source Unclear CVC in situ/ Inflammation at intravascular catheter insertion site/IV Drug Use/ Risk factors for MRSA |
Give antibiotics immediately |
||
|
Piperacillin/tazobactam IV 4.5g every 6 hours +
Gentamicin IV one dose per GAPP App calculator. See footnote 1 re further doses and monitoring. See footnote 2 re use in pregnancy. + Vancomycin IV infusion, dose per GAPP App calculator. See footnote 1 re review and monitoring.
See footnote 3 re sepsis in pregnancy. |
CefTRIAXone IV 2g every 24 hours + Gentamicin IV one dose per GAPP App calculator. See footnote 1 re further doses and monitoring. See footnote 2 re use in pregnancy. + Vancomycin IV infusion, dose per GAPP App calculator. See footnote 1 re review and monitoring. If pregnant or suspected intra-abdominal source: Add Metronidazole IV 500mg every 8 hours
See footnote 3 re sepsis in pregnancy. |
||
|
1 Review need for ongoing Gentamicin and Vancomycin on a daily basis. Continue with once daily Gentamicin dosing ONLY if Consultant / Specialist Registrar recommended. For advice on monitoring see Gentamicin & Vancomycin Dosing & Monitoring section. 2 Gentamicin & Ciprofloxacin are recommended in pregnancy when benefit outweighs risk. 3 For full detailed guidance on the management of sepsis in a pregnant patient, see WAC Directorate Guideline on the Management of Suspected Sepsis and Sepsis in Obstetric Care (QPulse CLN-OGCP-218). Discuss with Obstetrics. |
|||
Refs:
- Surviving Sepsis Campaign International Guidelines for Management of Sepsis and Septic Shock 2021
- NCEC Sepsis Management National Clinical Guideline No. 6 2014
- The Sanford Guide to Antimicrobial Therapy Digital Update Oct 2023
- HSE Sepsis programme documents and resources: https://www.hse.ie/eng/about/who/cspd/ncps/sepsis/resources/
Suspected Meningococcaemia (without features of meningitis)
Suspected Meningococcaemia (without features of meningitis)
- Discussion with Microbiology or Infectious Diseases recommended.
- When infection with susceptible N. meningitidis is confirmed, therapy with Benzylpenicillin alone is appropriate.
- Chloramphenicol is available in the Emergency Department and in the Pharmacy Department. Meropenem may be an alternative to chloramphenicol in patients with a history of penicillin anaphylaxis, as recommended in Irish guidelines, with close monitoring for cross-sensitivity e.g. in ICU.
- See Appendix 3 for management of contacts. Empiric Antibiotics for Suspected Meningococcaemia
|
Infection |
1 st Line Antibiotics |
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
Comment |
|
See penicillin hypersensitivity section for further information |
||||
|
Suspected Meningoccaemia (without features of meningitis) |
CefTRIAXone IV 2g every 12 hours |
CefTRIAXone IV 2g every 12 hours |
Give first dose Chloramphenicol IV 25mg/kg and IMMEDIATELY contact Microbiology or Infectious Diseases to discuss options. Discuss need for nasopharyngeal eradication for the patient with Microbiology or Infectious Diseases |
Duration 7 days |
Skin and Soft Tissue
Skin and Soft Tissue Infections
Skin and Soft Tissue Infections
-
The regimens below may NOT cover Multi-drug Resistant Organisms (MDRO) in all cases. Vancomycin may be required in addition. See note on MDRO .
-
Blood cultures should be performed before starting antimicrobial treatment if at all possible for a patient with a severe infection, especially if the patient is systemically ill.
-
Please avoid the prescription of antibiotics and submission of swabs for uninfected ulcers.
-
For suspected Orbital and Periorbital Cellulitis consult Ophthalmology urgently.
|
Empiric Antibiotics for Skin and Soft Tissue Infections |
|||||
|
Infection |
1 st Line Antibiotics |
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
Comment |
|
|
See penicillin hypersensitivity section for further information |
|||||
|
The regimens below may NOT cover Multi-drug Resistant Organisms (MDRO) in all cases. Vancomycin may be required in addition. See note on MDRO
|
|||||
|
Cellulitis/ Wound Infection (Including initial treatment of Mastitis)
NB: If treating Mastitis or Breast Abscess in the Lactating Woman, consultation with Obstetrics advised. See full detailed guidance – including treatment duration - in WAC Directorate Guideline on the Management of Mastitis and Breast Abscess in the Lactating Woman (QPulse CLN-OGCP-275)
|
Mild
Flucloxacillin PO 500mg – 1g 1 every 6 hours |
CefALEXin PO 500mg every 6 hours |
Clindamycin PO 450mg every 6 hours |
Duration for mild infection 5 days |
|
|
Moderate to severe Flucloxacillin IV 2g every 6 hours |
CefAZOLin (Unlicensed) IV 2g every 8 hours |
Vancomycin IV infusion, dose per GAPP App calculator. See footnote 2 re monitoring. |
Duration for moderate or severe infection 7 to 10 days |
||
|
Severe with incipient necrotising fasciitis Flucloxacillin IV 2g every 6 hours + Clindamycin 3 IV 600mg every 8 hours |
Vancomycin IV infusion, dose per GAPP App calculator. See footnote 2 re monitoring. + Clindamycin 3 IV 600mg every 8 hours Discuss with Microbiology or Infectious Diseases |
||||
|
For severe, if involving abdominal wall or groin or water exposure, consider adding Ciprofloxacin 3 IV 400mg every 12 hours |
|||||
|
Diabetic Foot Infection (without osteomyelitis )
Consider referral to Diabetic Foot Team (ENDF) |
Mild
Co-amoxiclav PO 625mg every 8 hours |
Clindamycin PO 450mg every 6 hours |
Duration: Minimum 7 days for mild infection 10 to 14 days in Moderate to Severe infection.
May require up to 3 weeks for severe infection. |
||
|
Moderate Co-amoxiclav IV 1.2g every 8 hours |
Clindamycin 3 IV 600mg every 8 hours + Ciprofloxacin 3 IV 400mg every 12 hours Monitor for diarrhoea |
||||
|
Severe Piperacillin/tazobactam IV 4.5g every 8 hours
|
Vancomycin IV infusion, dose per GAPP App calculator. See footnote 2 re monitoring. + Clindamycin 3 IV 600mg every 8 hours + Ciprofloxacin 3 IV 400mg every 12 hours |
||||
|
Discuss severe infections with Microbiology or Infectious Diseases. Higher doses may be indicated. |
|
||||
|
Necrotising fasciitis/gas gangrene (Group A Streptococcal infection)
Immediate surgical debridement is essential
Discuss immediately with Microbiology or Infectious Diseases
|
Flucloxacillin IV 2g every 4 hours + Benzylpenicillin IV 2.4g every 4 hours + Clindamycin IV 1.2g every 6 hours
For necrotising fasciitis of the abdominal wall or groin Consider adding Ciprofloxacin 3 IV 400mg every 8 hours + Metronidazole IV 500mg every 8 hours |
Discuss with Microbiology or Infectious Diseases
Consider Vancomycin IV infusion, dose per GAPP App calculator. See footnote 2 re monitoring. + Clindamycin 3 IV 1.2g every 6 hours + Ciprofloxacin 3 IV 400mg every 8 hours Monitor for diarrhoea
|
Usual duration 14 days |
||
|
Orbital and Periorbital Cellulitis Treat non-orbital facial cellulitis as cellulitis
Discuss with Ophthalmology
|
CefTRIAXone IV 2g every 24 hours + Metronidazole IV 500mg every 8 hours
Addition of Flucloxacillin IV 2g every 6 hours may be considered if S. aureus suspected
|
CefTRIAXone IV 2g every 24 hours + Metronidazole IV 500mg every 8 hours
Addition of Vancomycin IV infusion, dose per GAPP App calculator may be considered if S. aureus suspected. See footnote 2 re review and monitoring.
|
Vancomycin IV infusion, dose per GAPP App calculator. See footnote 2 re monitoring. + Clindamycin 3 IV 600mg every 8 hours + Ciprofloxacin 3 IV 400mg every 12 hours Monitor for diarrhoea Discuss with Microbiology or Infectious Diseases |
Duration 10 to 14 days |
|
|
1 The upper dose of Flucloxacillin PO 1g four times a day is unlicensed 2 Review need for ongoing vancomycin on a daily basis. For advice on monitoring see Vancomycin Dosing & Monitoring section. 3 Switch from IV to oral clindamycin (450mg every 6 hours) & from IV to oral ciprofloxacin (500mg every 12 hours) as soon as possible |
|||||
Refs:
- IDSA Guidelines for Diagnosis & Management of Skin & Soft-Tissue Infections 2014 Update. Clin Infect Dis 2014
- Guidelines on the diagnosis and treatment of foot infection in persons with diabetes IWGDF/IDSA 2023 https://iwgdfguidelines.org/infection-guideline-2023/
- NICE Guideline Diabetic foot problems: prevention and management 2015. Updated 2019 https://www.nice.org.uk/guidance/ng19
- Lehman. Flucloxacillin alone or combined with benzylpenicillin to treat lower limb cellulitis: a randomised controlled trial. Emerg Med J 2005;22:342-34 6
- Pham et al. 2022. Moderate to Severe Soft Tissue Diabetic Foot Infections. A Randomized, Controlled, Pilot Trial of Post-debridement Antibiotic Treatment for 10 versus 20 days. Annals of Surgery. Vol 276, number 2 233-238.
- Gariani et al. 2021. Three Weeks Versus Six Weeks of Antibiotic Therapy for Diabetic Foot Osteomyelitis: A Prospective, Randomized, Non inferiority Pilot Trial. Clinical Infectious Diseases. 73. E1539-154
Bites, Animal and Human, Prophylaxis and Treatment
Bites, Animal and Human, Prophylaxis and Treatment
- This provides recommendations on choice of antibiotic prophylaxis of bite wounds. It is not a comprehensive guide to the care of bite wounds. Depending on the nature of the injury, the type of bite, the country in which the bite occurred and previous immunization history issues such as prophylaxis against HIV virus infection and immunization against tetanus, hepatitis B and rabies may all merit consideration in addition to the issue of antibiotic prophylaxis addressed here. Refer to HSE immunisation guideline chapters on tetanus and rabies for risk assessment and management ( https://www.hiqa.ie/areas-we-work/national-immunisation-advisory-committee/immunisation-guidelines-ireland ). Refer to Emergency management of Injuries (EMI) guidance for assessment of risk of bloodborne viruses ( https://www.hpsc.ie/a-z/EMIToolkit/ )
- Application of topical antiseptics is NOT of value and should be avoided. Sterile water is appropriate for wound irrigation.
- Antibiotic prophylaxis is generally NOT appropriate for animal bites more than 2 days old OR human bites more than 3 days old at time of presentation.
-
Antibiotic prophylaxis
IS
appropriate for:
- All human bites less than 3 days old, all cat bites less than 2 days old and other animal bites less than 2 days old to the hand, foot, genitals and face; puncture or crush wounds; wounds that require surgical debridement or involving joints, tendons, ligaments or fractures.
- Wounds that have undergone primary closure.
- People at risk of serious wound infection (e.g. those who are immunocompromised, diabetic, asplenic or cirrhotic).
- People with a prosthetic valve or prosthetic joint.
- In the case of bites from monkeys seek to get as much information as possible about the species of monkey and discuss promptly with Microbiology or Infectious Diseases.
- If there are signs of infection, the issue is one of treatment rather than prophylaxis. In the absence of previous appropriate prophylaxis the regimens below are generally appropriate for treatment of infected bites; however the dose, route of administration, duration and choice of agents may require adjustment based on severity of infection.
|
Empiric Antibiotics for Animal and Human Bites |
|||
|
Infection |
1 st line Antibiotics |
In penicillin allergy |
Comment
|
|
Animal & human bites, prophylaxis and treatment |
Co-amoxiclav PO 625mg every 8 hours
|
Metronidazole PO 400mg every 8 hours + Doxycycline PO 100mg every 12 hours Avoid doxycycline in pregnancy or breastfeeding. Discuss with Micro/ID |
Duration: Prophylaxis - 3 days Treatment - 7 days Consider need for IV therapy or longer duration if severe infection
|
Refs:
- HSE. Bites (Human/Dog/Cat . Antibioticprescribing.ie April 2024
- NICE guideline. Human and animal bites: antimicrobial prescribing. November 2020.
Throat
Throat
- Antibiotic therapy is generally NOT indicated for acute pharyngitis/tonsillitis in the community as most throat infections are viral.
- This recommendation is for patients requiring hospitalisation.
- Avoid Amoxicillin and co-amoxiclav if glandular fever is considered likely.
- If condition is considered life threatening treat as for acute epiglottitis.
|
Empiric Antibiotics for Throat Infections |
||||
|
Infection |
1 st Line Antibiotics |
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
Comment |
|
See penicillin hypersensitivity section for further information |
||||
|
Acute Pharyngitis/ Tonsillitis
|
Benzylpenicillin IV 1.2g every 4 hours
If there is a good response to initial IV therapy consider switch to oral therapy with: Phenoxymethylpenicillin (Calvepen) PO 666mg every 6 hours OR Amoxicillin PO 500mg every 8 hours |
CefTRIAXone IV 2g every 24 hours
|
Clindamycin IV 600-900mg every 8 hours + Consider add Vancomycin IV infusion, dose per GAPP App calculator pending culture results. See footnote* re monitoring Discuss with Micro/ID |
Duration 10 days. Consider switch to oral therapy if good response to initial IV therapy If failure to respond to initial therapy consider Co-amoxiclav IV 1.2g every 8 hours (if no allergy to penicillin).
|
|
Peritonsillar abscess Discuss with ENT re early surgical drainage of abscess |
Co-amoxiclav IV 1.2g every 8 hours |
CefTRIAXone IV 2g every 24 hours + Metronidazole IV 500mg every 8 hours |
Clindamycin IV 600-900mg every 8 hours + Consider add Vancomycin IV infusion, dose per GAPP App calculator pending culture results. See footnote* re monitoring
Discuss with Micro/ID |
Duration usually 7 days. Consider oral switch when appropriate -discuss with Micro/ID. |
|
Severe Acute Epiglottitis Discuss with Microbiology or Infectious Diseases |
CefTRIAXone IV 2g every 24 hours |
CefTRIAXone IV 2g every 24 hours |
Vancomycin IV infusion, dose per GAPP App calculator. See footnote* re monitoring. + Ciprofloxacin IV 400mg every 12 hours
|
Optimal duration of treatment not established. Generally treat for 7-10 days. Longer duration may be indicated in selected patients. Consider oral switch when appropriate - discuss with Microbiology or Infectious Diseases. |
|
* For advice on monitoring see Vancomycin Dosing & Monitoring section. |
||||
Refs:
1. The Sanford Guide to Antimicrobial Therapy Digital App accessed online January 2024
2. Clinical Practice Guideline for the Diagnosis and Management of Group A Streptococcal Pharyngitis: 2012 Update by the Infectious Diseases Society of America
3. https://www.uptodate.com/contents/epiglottitis-supraglottitis-management#H18
Urinary Tract
Urinary Tract Infections
Urinary Tract Infections
- Non-pregnant patients with asymptomatic bacteruria do NOT require antibiotic treatment.
- Bacteriuria in a patient with an indwelling urinary catheter is NOT an indication for treatment unless there are specific clinical features of infection. Removal of the urinary catheter at the earliest possible time is the best approach to dealing with catheter-associated bacteriuria.
- A practice of routine antimicrobial prophylaxis with gentamicin or other agents at the time of catheterisation is NOT appropriate. See surgical prophylaxis section for note related to recent urological surgery .
- Multi-drug resistant organisms (MDRO) are relatively common in patients with UTI from a nursing home setting and increasingly in other patients. Review recent previous urine culture and sensitivity . See note on ESBL and MDRO .
- Check if any recent GP urine culture and sensitivities from iLab or contact the GP
- These are summary empiric antibiotic choices. Full detailed Women’s and Children’s (WAC) Group Management of Urinary Tract Infections (UTI’s) in Pregnancy are available on QPulse.
|
Empiric Antibiotics for Urinary Tract Infections |
||||
|
Infection
|
1 st Line Antibiotics |
Comment |
Duration |
|
|
The regimens below may NOT cover Multi-drug Resistant Organisms (MDRO) in all cases. See note on MDRO |
||||
|
Cystitis/Lower UTI
|
Nitrofurantoin PO 50mg every 6 hours Avoid nitrofurantoin if eGFR <45 ml/min/1.73 m2. When potential benefit outweighs risk, it may be used with caution if the eGFR is 30–44 ml/min/1.73 m2 for a short course only (3–7 days) |
Adjust initial treatment based on culture & sensitivity results. If eGFR<30ml/ min/1.73m 2 , discuss patients with Microbiology or Infectious Diseases If pregnant , see WAC Directorate Management of Urinary Tract Infections in Pregnancy (QPulse CLN-OGCP-227)
|
Duration for non-pregnant women : 3 days for nitrofurantoin (7 days in males) |
|
|
Infection |
1 st Line Antibiotics |
Penicillin allergy: delayed onset non-severe reaction |
Penicillin allergy: immediate or severe delayed reaction |
Duration |
|
See penicillin hypersensitivity section for further information |
||||
|
Pyelonephritis or Complicated UTI Non-pregnancy |
Piperacillin/tazobactam IV 4.5g every 8 hours + Gentamicin IV one dose per GAPP App calculator. See footnote* re further doses and monitoring.
If patient is septic and/or acutely unwell discuss with Microbiology or Infectious Diseases |
CefTRIAXone IV 2g every 24 hours
Add Gentamicin IV IF Sepsis . Give one dose per GAPP App calculator. See footnote* re further doses and monitoring. |
Ciprofloxacin IV 400mg (or PO 500mg) every 12 hours (consider oral route from outset). (See Fluoroquinolone warning ) Add Gentamicin IV IF Sepsis. Give one dose per GAPP App calculator. See footnote* re further doses and monitoring. |
Minimum duration of treatment is 10 days.
Longer duration may be necessary in males-discuss with Microbiology or Infectious Diseases
7 days if therapy is with Ciprofloxacin
Consider switch to oral therapy if good early clinical response to IV therapy. |
|
Acute Pyelonephritis in pregnancy |
For full detailed guidance see Women’s and Children’s (WAC) Group Management of Urinary Tract Infections (UTI’s) in Pregnancy (QPulse CLN-OGCP-227) |
Duration as per QPulse CLN-OGCP-227 |
||
|
CefTRIAXone IV 2g every 24 hours
Add Gentamicin IV IF Sepsis . Give one dose per GAPP App calculator (use booking weight). See footnote* re further doses and monitoring. See footnote^ re use in pregnancy.
|
Gentamicin IV every 24 hours, dose per GAPP App calculator (use booking weight). See footnote* re review and monitoring. See footnote^ re use in pregnancy. + Either (depending on Group B Streptococcus susceptibility result if available) Vancomycin IV infusion, dose per GAPP App calculator (use booking weight). See footnote* re review and monitoring. OR Clindamycin IV 900mg every 8 hours
Give first doses, THEN IMMEDIATELY discuss with Microbiology or Infectious Diseases to discuss further therapy. |
|||
|
^ Gentamicin is recommended in pregnancy when benefit outweighs risk. * Review need for ongoing Gentamicin and Vancomycin on a daily basis. Continue with once daily Gentamicin dosing ONLY if Consultant /Specialist Registrar recommended. Up to three once daily doses of Gentamicin may be indicated for pyelonephritis. For advice on monitoring see Gentamicin & Vancomycin Dosing & Monitoring section. |
||||
Refs:
- IDSA/ESCMID Guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women. Clin Infect Dis 2011;52:e103-e120
- SIGN160 : Management of suspected bacterial lower urinary tract infection in adult women. Sept 2020
- Women’s and Children’s (WAC) Group Management of Urinary Tract Infections (UTI’s) in Pregnancy (QPulse CLN-OGCP-227)
- NICE guidelines NG 109 : Urinary tract infection (lower): antimicrobial prescribing. Published 31 October 2018
- NICE guidelines NG 111 : Pyelonephritis (acute): antimicrobial prescribing. Published 31 October 2018
- Antimicrobial for 7 or 14 Days for febrile Urinary Tract Infection in Men: a multicentre noninferiority double-blind, placebo-controlled, randomized clinical trial. Clin Infect Dis 2023:76 2154-2162
Prophylaxis of Recurrent Urinary Tract Infections
Prophylaxis of Recurrent Urinary Tract Infections
Discussion with Microbiology or Infectious Diseases is recommended. In the absence of a correctable anatomical or other predisposing factor for recurrent UTI, prophylaxis for a period of 3 to 6 months may be considered. There is limited evidence of any additional benefit from such prophylaxis beyond 6 months. In general the most appropriate agent for prophylaxis is nitrofurantoin PO 50mg to 100 mg at night.
CAUTION: Continuation of nitrofurantoin is very rarely justified and if considered should be discussed with Microbiology or Infectious Diseases. Monitor lung and liver function in patients on long-term nitrofurantoin therapy. Avoid in renal impairment (eGFR less than 45ml/min/1.73m 2 when used as prophylaxis).

