Paediatric Guidelines
Download / Print Section as PDFThe LH Paediatric Antimicrobial Guidelines were adapted with kind permission from the Children's Health Ireland (CHI) Antimicrobial Guidelines 2024. Antimicrobial choices and management advice have been amended where necessary to reflect local antimicrobial resistance data and the type of patients and infections encountered in LH.
Please see BNFc and/or CHI 'Clinibee' App for paediatric antimicrobial dosing, unless dose already specified in this guideline.
Paediatrics - Evaluating for antibiotic allergy before prescribing antimicrobials
Background:
Before prescribing any antimicrobial agent, a history of possible contraindications, adverse reactions, allergies, must be sought. Rashes, including urticarial rashes are common occurrences in childhood febrile illnesses. Over 90% of reported paediatric “allergic” reactions to antimicrobials cannot be repeated or were merely well described side effects such as penicillin induced diarrhoea or macrolide induced nausea. Unnecessary use of alternative antibiotics increases the risk of development of antimicrobial resistance. Incorrect labelling of antibiotic allergy can lead to unnecessary use of more toxic alternative antimicrobials and increase hospital stay.
Types of allergic reactions:
Immediate hypersensitivity reactions (Type 1)
Type 1 reactions are IgE-mediated and occur <1 hour post dose. Clinical signs include urticarial or pruritic rash, angioedema, rhinitis, respiratory and or cardiovascular compromise.
Delayed Hypersensitivity reactions (Types II, III, IV)
Type II reactions are IgG mediated and occur > 72 hours post dose. These are not true ‘allergic reactions’ however these reactions should lead to avoidance of future use of the suspected drug. Common manifestations of this type of reaction include haemolytic anaemia, neutropenia or thrombocytopenia.
Type III reactions are generally associated with immune complex deposition and complement activation (e.g. serum sickness like reaction to cefaclor, glomerulonephritis).
Type IV reactions are the most common drug hypersensitivity reactions encountered. These are not antibody mediated but relate to T cell activity. The skin is most often involved in generalised maculopapular eruptions (e.g. beta- lactam related rashes typically developing after a number of days on treatment).
Taking a drug allergy history:
The parent should be asked to describe the previous reaction, including timing of the reaction, the type of rash, distribution and how long it took to resolve. Photos if available should be reviewed. Maculopapular rashes that develop in young children on day 3 or 4 post commencing a course of oral antibiotic and resolve quickly are very unlikely to indicate future risk of severe allergic reaction.
Red flags: (Symptoms more likely to indicate risk of future reactions)
- History of angioedema
- History of breathing difficulties
- History suggestive of cardiovascular compromise
The symptoms listed above are strongly suggestive of a previous Type 1 reaction and thus future prescribing would be contraindicated.
- Joint swelling
A history of joint swelling may indicate serum sickness like reaction. Further administration can trigger a Type 1 reaction. In the first instance avoidance is advised.
- History of hospitalisation due to previous drug eruption.
- History of skin peeling or desquamation
- History of bruising (vasculitis)
- History of involvement of mucous membranes
- History of internal organ involvement, abnormal blood parameters
The symptoms listed suggest a previous severe cutaneous adverse reaction (SCAR). In this case re-prescribing of the suspected agent is contraindicated as SCARs carry a mortality rate of 10%.
Management Options:
|
No |
Conclusion |
Outcome |
|
1 |
History of adverse reaction is not consistent with drug allergy |
The recommended antimicrobial can be prescribed |
|
2 |
History suggests a probable drug allergy |
Choose alternative antimicrobial and consider referral to the allergy team for consideration for elective drug provocation test |
|
3 |
History indicates a probable allergic reaction but alternative antimicrobial choices are limited |
Choose alternative antimicrobial in the short term and consider referral to the allergy team for possible formal drug provocation test |
|
4 |
History clearly suggests previous Type 1 allergic reaction but alternative antimicrobial choices are limited |
Choose alternative antimicrobial and consider referral to the allergy team regarding appropriateness of desensitisation |
|
5 |
The history is suggestive of a previous SCAR |
Retrial of antibiotic completely contraindicated. Caution re: cross-reactivity. Discuss antibiotic selection with Micro/ID team. |
|
6 |
Patient has previously confirmed by the allergy team to have a drug allergy |
Choose alternative antibiotic. |
Note: Discussion with the ID/Microbiology may be necessary, in order to choose the most appropriate alternative antimicrobial.
Recording adverse drug reactions:
If the clinician determines from the history that use of a particular antimicrobial is contraindicated, this should be documented:
- On the most recent drug chart, dated and signed
- A more detailed note should be recorded in the medical notes documenting all aspects of the history that lead to the decision to avoid the antibiotic
- A follow up plan should be made and documented in the medical notes: see above re management options.
Essential data to record in the medical notes:
- The date of the reaction
- The time of onset with relation to the most recent course of antibiotics
- If multiple antibiotics have been prescribed, document the date of onset of each one
-
Record all symptoms and clinical signs including those that may not be (at first glance) involved
- Rash: Include its distribution, characteristics, mucous membrane involvement/not, obvious areas that are spared
- Presence or absence of lymphadenopathy (check all sites)
- Evidence of internal organ involvement
- Presence or absence of fever
- Also record any abnormal laboratory indices: LFTs, eosinophilia, cytopenia etc.
Choosing alternative antibiotics:
- Patients with a history of Type 1 reactions to penicillin or amoxicillin are likely to tolerate monobactams and carbapenems.
- Patients with a history of Type 1 reactions to amoxicillin are likely to react to cephalosporins with a similar side chain on the β-lactam ring i.e. 1st and 2nd generation cephalosporins. These should be avoided. Other cephalosporins (3rd, 4th and 5th generation) with different side chains are more likely to be tolerated in penicillin allergic individuals. If the initial reaction was severe (anaphylaxis) discuss with allergy team before administration.
- Patients with a history of delayed reactions such as morbilliform or maculopapular rash may also tolerate 1st and 2nd generation cephalosporins.
- Patients with a history of SCARs (SJS, TENS, DRESS) or haemolytic anaemia should not be commenced on a beta-lactam without discussion with allergy/ID/Micro teams.
Paediatric Empiric Treatment Guidelines
Paediatrics - Bone and Joint Infections
|
Infection |
|
Paediatrics - Acute Osteomyelitis or Septic Arthritis |
|
Likely Organisms |
|
Child < 3 months S. aureus, Group B Streptococcus, H. influenzae & other gram negative bacilli Child > 3 months S. aureus, Group A Streptococcus, Kingella kingae if ≤ 5 years, H. influenzae in septic arthritis in unvaccinated individuals |
|
Empiric Antimicrobial Treatment |
|
Child < 3 months Cef-O-taxime IV plus Flucloxacillin IV plus Gentamicin IV Child > 3 months to < 5 years Cef-AZ-olin IV 50mg/kg TDS (max 6g/day)
Child > 5 years
Flucloxacillin IV OR Cef-AZ-olin IV 50mg/kg TDS (max 6g/day) |
|
Duration of Treatment |
|
Contact Consultant Microbiologist for advice. |
|
IV to Oral Switch |
|
|
Comments |
|
Kingella kingae susceptible to cephalosporins but not to flucloxacillin. |
|
Infection |
|
Paediatrics - Osteomyelitis in sickle cell disease or galactosaemia |
|
Likely Organisms |
|
S. aureus, Group A Streptococcus, Salmonella |
|
Empiric Antimicrobial Treatment |
|
Flucloxacillin IV plus Cef-TRI-axone IV OR Cef-O-taxime IV |
|
Duration of Treatment |
|
Contact Consultant Microbiologist for advice. |
|
IV to Oral Switch |
|
| Infection |
| Paediatrics - Osteomyelitis following penetrating injury to foot |
|
Likely Organisms |
|
Pseudomonas aeruginosa, S. aureus, Streptococci |
|
Empiric Antimicrobial Treatment |
|
Piperacillin/tazobactam IV +/- Gentamicin IV |
|
Duration of Treatment |
|
Contact Consultant Microbiologist for advice. |
|
IV to Oral Switch |
|
|
Infection |
|
Paediatrics - Osteomyelitis in an immunocompromised child |
|
Comments |
|
Contact Consultant Microbiologist for advice |
|
Infection |
|
Paediatrics - Chronic Osteomyelitis |
|
Comments |
|
Contact Consultant Microbiologist for advice. The antimicrobial treatment of ALL cases of chronic osteomyelitis should be discussed with the Consultant Microbiologist or ID Consultant. Bone specimen should be obtained for culture and sensitivity prior to initiation of antimicrobials – use susceptibilities to guide choice. |
Paediatrics - Central Nervous System Infections
|
Infection |
|
Paediatrics - Acute Bacterial Meningitis: Child < 8 weeks Excludes neutropenic sepsis |
|
Likely Organisms |
|
Child < 8 weeks (chronological age) Group B Streptococcus, E. coli, Listeria monocytogenes, N. meningitidis, S. pneumoniae |
|
Empiric Antimicrobial Treatment |
|
For pre-term infants or previous NICU admission, refer patient to Neonatology / Microbiology. Child < 8 weeks (chronological age) Cef-O-taxime IV Plus Amoxicillin IV Plus consider (see comments below): +/- Gentamicin IV +/- Vancomycin IV +/- Aciclovir IV Plus contact Microbiology if recent foreign travel for mother or baby in case of potential for colonisation with resistant organism. Plus If > 6 weeks old, add dexamethasone 0.15 mg/kg (max 10 mg) 6 hourly IV for 4 days if H. influenzae / S. pneumoniae meningitis is suspected or confirmed as it may reduce long-term complications. In this case, ideally it should be given just before or within 1 hour of the first dose of antibiotics . Consult Microbiology.
Add Gentamicin if : • Severe sepsis/ haemodynamically unstable • Requiring inotropes/critical care • Likely resistant organisms e.g., frequent or prolonged hospitalisation; >48 hours following admission; recent foreign travel for mother or baby.
Add Vancomycin if: • MRSA positive • Recent travel outside of Ireland for mother or baby • Prolonged antibiotics in past 3 months • Concern about infected prosthetic material e.g. PICC line in-situ.
Add Aciclovir if clinical features of HSV.
Add Clindamycin if suspected staphylococcal/streptococcal toxic shock.
If suspected abdominal source, please see monograph for Paediatric Intra-Abdominal Infections . |
|
Duration of Treatment |
|
For uncomplicated meningitis where causative organism known:
For culture and PCR negative suspected bacterial meningitis:
Longer durations may be required if persistent fever or other complications. |
|
IV to Oral Switch |
|
Continue IV therapy for entire duration of treatment. |
|
Comments |
Public Health notification required for meningitis caused by N. meningitidis, H. influenzae, S. pneumoniae, Listeria spp. and viral meningitis. |
|
Infection |
|
Paediatrics - Acute Bacterial Meningitis: Child > 8 weeks Excludes neutropenic sepsis |
|
Likely Organisms |
|
Child > 8 weeks N. meningitidis, S. pneumoniae, H. Influenzae, Group B Streptococcus if ≤12 weeks |
|
Empiric Antimicrobial Treatment |
|
Child > 8 weeks Cef-O-taxime IV Plus consider (see comments below): +/- Gentamicin IV +/- Vancomycin IV +/- Aciclovir IV Plus contact Microbiology if recent foreign travel for mother or baby in case of potential colonisation with resistant organism. Plus If > 6 weeks old, add dexamethasone 0.15 mg/kg (max 10 mg) 6 hourly IV for 4 days if H. influenzae / S. pneumoniae meningitis is suspected or confirmed as it may reduce long-term complications. In this case, ideally it should be given just before or within 1 hour of the first dose of antibiotics . Consult Microbiology.
Add Gentamicin if : • Severe sepsis/ haemodynamically unstable • Requiring inotropes/critical care • Likely resistant organisms e.g., frequent or prolonged hospitalisation; >48 hours following admission; recent foreign travel for mother or baby.
Add Vancomycin if: • MRSA positive • Recent travel outside of Ireland for mother or baby • Prolonged antibiotics in past 3 months • Concern about infected prosthetic material e.g. PICC line in-situ.
Add Aciclovir if clinical features of HSV.
Add Clindamycin if suspected staphylococcal/streptococcal toxic shock.
If suspected abdominal source, please see monograph for Paediatric Intra-Abdominal Infections . |
|
Duration of Treatment |
|
For uncomplicated meningitis where causative organism known:
For culture and PCR negative suspected bacterial meningitis:
Longer durations may be required if persistent fever or other complications. |
|
IV to Oral Switch |
|
Continue IV therapy for entire duration of treatment. |
|
Comments |
Public Health notification required for meningitis caused by N. meningitidis, H. influenzae, S. pneumoniae, Listeria spp. and viral meningitis. |
|
Infection |
|
Paediatrics - Brain Abscess |
|
Likely Organisms |
|
α-haemolytic streptococci, anaerobes, S. aureus, Pseudomonas aeruginosa |
|
Empiric Antimicrobial Treatment |
|
Cef-O-taxime IV plus Metronidazole IV plus Vancomycin IV If Pseudomonas suspected (e.g. abscess related to chronic ear focus or chronic sinusitis, or immunocompromised host): Replace Cef-O-taxime IV with Cef-TAZ-idime IV |
|
Duration of Treatment |
|
Consult Microbiology. |
|
IV to Oral Switch |
|
Continue IV therapy for entire duration of treatment. |
|
Comments |
|
Empiric therapy should be changed to directed therapy as soon as the organism and susceptibility patterns are known. |
|
Infection |
|
Paediatrics - Encephalitis |
|
Likely Organisms |
|
Herpes simplex virus (HSV), Enteroviruses, Influenza, Mycoplasma pneumoniae |
|
Empiric Antimicrobial Treatment |
|
Aciclovir IV To be commenced only if clinical features of encephalitis e.g.:
A macrolide (azithromycin PO or clarithromycin IV) may be added if history suggestive of mycoplasma infection. |
|
Duration of Treatment |
|
Treat for 21 days, or until HSV infection has been excluded (negative CSF HSV PCR at >72hrs following onset of neurological symptoms AND low clinical suspicion of HSV) |
|
IV to Oral Switch |
|
Continue IV therapy for entire duration of treatment. |
|
Comments |
|
Ensure adequate hydration while on aciclovir IV to prevent renal toxicity. Addition of Aciclovir not necessary in the absence of seizures or signs of encephalitis. Should be reviewed on a case by case basis depending on the clinical situation. |
Paediatrics - Dental Infections
|
Infection |
|
Paediatrics - Dental Infections |
|
Likely Organisms |
|
Anaerobes, Viridans streptococci |
|
Empiric Antimicrobial Treatment |
|
Mild: Amoxicillin PO If penicillin allergic: 1 st line: Metronidazole PO OR 2 nd line: Clindamycin PO
Severe: Seek Dental Consult Amoxicillin IV plus Metronidazole IV If penicillin allergic: Clindamycin IV |
|
Duration of Treatment |
|
Mild: Up to 5 days; review at 24 to 48 hours. Severe: 5 days - if definitive source control (e.g. removal of the causative tooth) is achieved, then discontinuation of antibiotic therapy can be considered before the 5 days is completed. |
| Comments |
|
Antibiotics are only required in the case of spreading infection (cellulitis, lymph node involvement, swelling) or systemic involvement (fever, malaise). Severe infection: significant trismus, extra oral swelling, eye closing, floor of mouth swelling, difficulty breathing, systemic symptoms or rapidly progressing spread of infection. For less acute dental indications, please follow HSE dental community guidelines located at www. antibioticprescribing.ie . |
Paediatrics - ENT Infections
|
Infection |
|
Paediatrics - Cervical Lymphadenitis |
|
Likely Organisms |
|
S. aureus, Group A Streptococcus, anaerobes, Group B Streptococcus or S. aureus if < 3 months old |
|
Empiric Antimicrobial Treatment |
|
Mild (outpatient): Cef-AL-exin PO OR Flucloxacillin PO OR Co-amoxiclav PO
Moderate to Severe (hospitalised): Cef-AZ-olin IV OR Flucloxacillin IV Plus Clindamycin PO or IV |
|
Duration of Treatment |
|
Mild to Moderate: 7 days. Severe: Duration as per Micro/ID. |
|
IV to Oral Switch |
|
Yes, when clinically appropriate. Cef-AL-exin is an appropriate PO switch for Cef-AZ-olin IV. |
|
Comments |
|
If suppuration present, may require incision & drainage, contact ENT. |
|
Infection |
|
Paediatrics - Epiglottis - Acute |
|
Likely Organisms |
|
S. pneumoniae, Group A and C Streptococcus, S. aureus (H. influenzae, now rare) |
|
Empiric Antimicrobial Treatment |
|
Cef-O-taxime IV |
|
Duration of Treatment |
|
7 to 10 days |
|
IV to Oral Switch |
|
Continue IV for entire duration of therapy. |
|
Infection |
|
Paediatrics - Mastoiditis - Acute |
|
Likely Organisms |
|
S. pneumoniae, Group A Streptococcus, S. aureus |
|
Empiric Antimicrobial Treatment |
|
Cef-O-taxime IV OR Cef-TRI-axone IV If known MRSA, contact Microbiology for advice. If history of recent antibiotic use and otorrhoea, may need P. aeruginosa cover, contact Microbiology for advice. |
|
Duration of Treatment |
|
14 days. |
|
IV to Oral Switch |
|
Yes, when clinically appropriate. |
|
Infection |
|
Paediatrics - Mastoiditis - Chronic |
|
Likely Organisms |
|
Often polymicrobial, anaerobes, S. aureus, Enterobacteriaceae, P. aeruginosa |
|
Empiric Antimicrobial Treatment |
|
Consult Microbiology Antimicrobial treatment should be guided by culture and sensitivity results. |
|
Duration of Treatment |
|
Duration to be decided on an individual case-by-case basis. Contact Microbiology for advice if required. |
|
IV to Oral Switch |
|
Yes, when clinically appropriate. |
|
Comments |
|
Antibiotic treatment should begin after drainage. Obtain samples before starting therapy. |
|
Infection |
|
Paediatrics - Otitis Externa |
|
Likely Organisms |
|
S. aureus, Pseudomonas aeruginosa, (Aspergillus) |
|
Empiric Antimicrobial Treatment |
|
Local cleaning and antiseptic application are often sufficient. Control seborrhoea with dandruff shampoo.
If antibiotic necessary: 1st line: Topical Ciprofloxacin ear drops
If systemic treatment required: Flucloxacillin PO OR Cefalexin PO If Pseudomonas isolated discuss with Microbiology. |
|
Duration of Treatment |
|
5 to 7 days |
|
Infection |
|
Paediatrics - Otitis Media- Acute |
|
Likely Organisms |
|
Often viral, H. influenzae (<5 years), S. pneumoniae, Group A Streptococcus, (Moraxella catarrhalis) |
|
Empiric Antimicrobial Treatment |
|
First episode : Amoxicillin PO Recurrent or failure to respond after 3 days : Co-amoxiclav PO OR Clarithromycin PO Severe, unresponsive to PO therapy: Cef-TRI-axone IV |
|
IV to Oral Switch |
|
Yes, when clinically appropriate. |
|
Duration of Treatment |
|
5 to 7 days. |
|
Comments |
|
Most cases, whether viral or bacterial, resolve spontaneously ; consider delaying antibiotic therapy for 48 hours in previously well children of > 2 years, and treating then if still symptomatic. Note on dose of oral antibiotic: Use the highest end of dose range where one exists. |
|
Infection |
|
Paediatrics - Peritonsillar Abscess, Retropharyngeal/Parapharyngeal Abscess |
|
Likely Organisms |
|
S. aureus, Group A Streptococcus, anaerobes |
|
Empiric Antimicrobial Treatment |
|
Cef-O-taxime IV Plus Clindamycin PO or IV |
|
Duration of Treatment |
|
Duration 10 to 14 days including IV to oral switch. Consider stopping clindamycin after 3 - 5 days. Duration depends on clinical response and drainage. If pus is drained send to the lab for culture and sensitivity. |
|
IV to Oral Switch |
|
Yes when clinically appropriate. |
|
Infection |
|
Paediatrics - Pharyngitis Or Tonsillitis |
|
Likely Organisms |
|
Viruses (most cases), Group A Streptococcus (S. pyogenes) |
|
Empiric Antimicrobial Treatment |
|
See comments. If treatment considered necessary: Phenoxymethylpenicillin PO OR Amoxicillin PO OR If penicillin allergic: Non-immediate non-severe penicillin hypersensitivity: Cef-AL-exin PO Immediate or severe penicillin hypersensitivity: Clarithromycin PO |
|
Duration of Treatment |
|
5 days. If severe / relapse /no clinical response / scarlet fever (Group A Strep positive throat swab cultures or rapid tests): 10 days. |
|
Comments |
|
Treat only if proven bacterial cause. Antibiotics make little difference to how long symptoms last or the number of people whose symptoms improve. In recurrent and/or refractory infection, consult Microbiology. |
|
Infection |
|
Paediatrics - Sinusitis - Acute |
|
Likely Organisms |
|
S. pneumoniae, Group A Streptococcus (Moraxella catarrhalis) |
|
Empiric Antimicrobial Treatment |
|
See comments. If treatment considered necessary: 1 st line: Amoxicillin PO 2 nd line: May switch to Co-amoxiclav PO if not responding |
|
Duration of Treatment |
|
5 days. |
|
Comments |
|
Most resolve spontaneously. Treat with antibiotics only if not resolving after 10 days. Give high dose in severe infection. |
|
Infection |
|
Paediatrics - Tracheitis - Bacterial |
|
Likely Organisms |
|
S. aureus, H. influenzae, M. catarrhalis, Group A Streptococcus |
|
Empiric Antimicrobial Treatment |
|
Cef-UR-oxime IV OR Co-amoxiclav IV |
|
Duration of Treatment |
|
7 to 10 days. |
|
IV to Oral Switch |
|
Yes, when clinically appropriate. N.B. Cef-UR-oxime PO is not recommended due to low oral bioavailability. |
|
Comments |
|
N.B. Take a sample for gram stain and culture before starting antibiotics. |
|
Infection |
|
Paediatrics - Tracheitis in a patient with tracheostomy |
|
Likely Organisms |
|
S. aureus, H. influenzae, M. catarrhalis, Group A Streptococcus, P. aeruginosa |
|
Empiric Antimicrobial Treatment |
|
Cef-UR-oxime IV OR Co-amoxiclav IV Add Ciprofloxacin* PO if Pseudomonas a consideration *Caution with Ciprofloxacin – risk of long-lasting and disabling adverse effects, mainly involving muscles, tendons and bones and the nervous system. Use with caution in patients pre-disposed to seizures, G6PD deficiency, myasthenia gravis and conditions (and other medication) predisposing to prolonged QT interval. |
|
Duration of Treatment |
|
7 days (or longer, depending on response) |
|
IV to Oral Switch |
|
Yes, when clinically appropriate. |
|
Comments |
|
N.B. Take a sample for gram stain and culture before starting antibiotics. |
Paediatrics - Eye Infections
|
Infection |
|
Paediatrics - Conjunctivitis |
|
Likely Organisms |
|
Viruses (most cases): Enteroviruses, Adenovirus, Herpes simplex virus Bacteria: H. influenzae, S. pneumoniae, M. catarrhalis |
|
Empiric Antimicrobial Treatment |
|
Child > 1 month Treat only if proven bacterial conjunctivitis. Most cases, whether viral or bacterial, resolve spontaneously. Topical chloramphenicol eye drops, continue for 48 hours after healing. Note - the previous warning associated with use of chloramphenicol eye drops in patients under 2 years of age has been reviewed and removed. |
|
Duration of Treatment |
|
5 – 7 days |
|
IV to Oral Switch |
|
N/A |
|
Comments |
|
Consult Microbiology if Group B Streptococcus is identified. |
|
Infection |
|
Paediatrics - Cellulitis: Pre-septal (Peri-Orbital) |
|
Likely Organisms |
|
S. aureus, Group A Streptococcus, Pneumococcus, H. influenzae in unvaccinated individuals |
|
Empiric Antimicrobial Treatment |
|
Mild Cases : Co-amoxiclav PO OR Cef-AL-exin PO Severe Cases : Cef-O-taxime IV |
|
Duration of Treatment |
|
10 to 14 days ( including IV to oral switch) |
|
IV to Oral Switch |
|
As per clinical response |
|
Comments |
|
In severe cases:
For children ill enough to require IV therapy, CT scan is recommended to determine:
Tailor therapy to the most appropriate agents based on culture and sensitivity results. |
|
Infection |
|
Paediatrics - Cellulitis: Post-septal (Orbital) |
|
Likely Organisms |
|
S. pneumoniae, S. aureus, aerobic gram negative bacilli, anaerobes |
|
Empiric Antimicrobial Treatment |
|
Cef-O-taxime IV plus Metronidazole IV |
|
Duration of Treatment |
|
14 to 21 days including IV to oral switch |
|
IV to Oral Switch |
|
As per clinical response |
|
Comments |
|
In severe cases:
For children ill enough to require IV therapy, CT scan is recommended to determine:
Tailor therapy to the most appropriate agents based on culture and sensitivity results. |
Paediatrics - Febrile Neutropenia
Please refer directly to Children's Health Ireland (CHI) 'Clinibee' Antimicrobial Guidelines app for guidance. Also, contact Microbiology for advice if needed.
Paediatrics - Fungal Infections
Please refer directly to Children's Health Ireland (CHI) 'Clinibee' Antimicrobial Guidelines app for guidance. Also, contact Microbiology for advice if needed.
Paediatrics - Gastrointestinal Infections
|
Infection |
|
Paediatrics - Acute Gastroenteritis |
|
Likely Organisms |
|
Usually viral |
|
Empiric Antimicrobial Treatment |
|
Antibiotic rarely if ever indicated. |
Paediatrics - Intra-abdominal Infections
|
Infection |
|
Paediatrics - Acute Abdominal Sepsis E.g.
|
|
Likely Organisms |
|
E. coli and other gram negative bacilli, anaerobes, Streptococci, Staphylococci |
|
Empiric Antimicrobial Treatment |
|
Piperacillin/tazobactam IV plus Gentamicin IV In infected ascites, if MRSA suspected: Add Vancomycin IV |
|
Duration of Treatment |
|
Minimum 10 to 14 days |
|
IV to Oral Switch |
|
Consult Microbiology |
|
Infection |
|
Paediatrics - Acute Appendicitis |
|
Likely Organisms |
|
E. coli and other gram negative bacilli, anaerobes, Streptococci especially S. milleri |
|
Empiric Antimicrobial Treatment |
|
Cef-UR-oxime IV plus Metronidazole IV +/- Gentamicin IV |
|
Duration of Treatment |
|
Uncomplicated appendix: No further antibiotic doses post-operatively. Perforated or appendix mass: 7 days (or longer if peritonitis suspected) |
|
IV to Oral Switch |
|
N.B. Cef-UR-oxime PO is not recommended due to low oral bioavailability. Change to oral cefaclor and metronidazole when child meets the COMS criteria for IV to oral switch . |
|
Infection |
|
Paediatrics - Enterocolitis (non C. difficile) or Faecal Peritonitis |
|
Likely Organisms |
|
E. coli and other gram negative bacilli, anaerobes, streptococci |
|
Empiric Antimicrobial Treatment |
|
Amoxicillin IV Plus Gentamicin IV Plus Metronidazole IV |
|
Duration of Treatment |
|
If source of faecal soiling of peritoneum has been sealed and no abscess, then treatment duration of 5 days is sufficient. |
|
Infection |
|
Paediatrics - Necrotising Enterocolitis |
|
Likely Organisms |
|
Multifactorial condition, organisms that may be involved include Staphylococci, Clostridium perfringens, Klebsiella spp. |
|
Empiric Antimicrobial Treatment |
|
Amoxicillin IV Plus Gentamicin IV Plus Metronidazole IV |
|
Duration of Treatment |
|
Minimum 10 to 14 days IV |
|
Comments |
|
If the child is known to be colonised with drug resistant organisms (e.g. ESBL or CRE), drug choices may need to be modified: Consult Microbiology. |
|
Infection |
|
Paediatrics - Toxic Megacolon in Inflammatory Bowel Disease |
|
Likely Organisms |
|
E. coli and other gram negative bacilli, anaerobes, Streptococci, Staphylococci, C. difficile |
|
Empiric Antimicrobial Treatment |
|
Treatment can vary depending on previous antibiotic therapy, risk of C. difficile etc. Consult Microbiology |
|
Duration of Treatment |
|
Consult Microbiology |
Paediatrics - Malaria
Paediatrics - Severe Malaria
|
Infection |
|
Paediatrics - Severe Malaria (warrants ICU admission) Severity indicators: Hyperparasitaemia > 5%, Neurological abnormality, renal impairment, acidosis, hypoglycaemia, respiratory distress, Hb <8g/dl, spontaneous bleeding/DIC, shock, haemoglobinuria . |
|
Likely Organisms |
|
Plasmodium falciparum most likely |
|
Empiric Antimicrobial Treatment |
|
1 st Line : Artesunate IV for at least 24 hours, duration of IV therapy based on clinical response Artesunate IV Dosing Regimen:
Once patient is clinically improved and IV to oral switch appropriate, complete treatment with: Artemether-Lumefantrine PO (Riamet®) - dose as per BNF for Children, dose given at 0h, 8h, 24h, 36h, 48h and 60h (total course given over 60 hours = 2.5 days) N.B. Please note the timing of Riamet® doses relates to time from time zero – see worked example below:
N.B. Contact Pharmacy Department prior to discharge to ensure continuity of supply as Riamet® is not readily available in the community
2 nd Line: IV Quinine no longer available (Jul 2019) |
| Comments |
|
References for Artesunate IV Dose:
|
Paediatrics - Uncomplicated Malaria
|
Infection |
|
Paediatrics - Uncomplicated Malaria: Plasmodium falciparum or species not identified |
|
Empiric Antimicrobial Treatment |
|
1st Line: Artemether-Lumefantrine PO (Riamet®) - dose as per BNF for Children, dose given at 0h, 8h, 24h, 36h, 48h and 60h N.B. Please note the timing of Riamet® doses relates to time from time zero – see worked example below:
N.B. Contact Pharmacy Department prior to discharge to ensure continuity of supply as Riamet® is not readily available in the community.
2nd Line: Atovaquone-Proguanil PO (Malarone / Malarone Paed ®) for 3 days |
|
Comments |
|
Consideration must be given to admit patient for a minimum of 24 hours. All confirmed or suspected cases must be discussed with Infectious Diseases/Microbiology before discharge.
|
|
Infection |
|
Paediatrics - Uncomplicated Malaria: Plasmodium malariae or knowlesi Acquired in any region |
|
Empiric Antimicrobial Treatment |
|
Chloroquine phosphate PO for 3 days |
| Comments |
| All confirmed or suspected cases must be discussed with Infectious Diseases/Microbiology before discharge . |
|
Infection |
|
Paediatrics - Uncomplicated Malaria: Plasmodium vivax or Plasmodium ovale |
|
Empiric Antimicrobial Treatment |
|
If acquired in any region except Papua New Guinea or Indonesia : Chloroquine Phosphate PO for 3 days Followed by Primaquine Phosphate* PO for 14 days
Plasmodium vivax acquired in Papua New Guinea or Indonesia (chloroquine-resistant) : 1 st Line: Artemether-Lumefantrine PO (Riamet®) - dose as per BNF for Children, dose given at 0h, 8h, 24h, 36h, 48h and 60h (total course given over 60 hours = 2.5 days) N.B. Please note the timing of Riamet® doses relates to time from time zero – see worked example below:
N.B. Contact Pharmacy Department prior to discharge to ensure continuity of supply as Riamet® is not readily available in the community Followed by Primaquine Phosphate* PO for 14 days
2nd Line: Atovaquone-Proguanil PO for 3 days PLUS Primaquine Phosphate* PO for 14 days |
|
Comments |
|
* Screen for G6PD deficiency before starting primaquine - primaquine is given to eradicate parasites in the liver and thus prevent relapse, it can be started in the follow-up OPD appointment once result of G6PD deficiency screen available:
All confirmed or suspected cases must be discussed with Infectious Diseases/Microbiology before discharge. |
Paediatrics - Respiratory Tract Infections
|
Infection |
|
Paediatrics - Aspiration Pneumonia – Community-acquired |
|
Likely Organisms |
|
Streptococci, oral flora including anaerobes, aerobic gram negative bacilli |
|
Empiric Antimicrobial Treatment |
|
Co- amoxiclav IV
If penicillin allergic: Co-trimoxazole IV Plus Metronidazole PO or IV |
|
Duration of Treatment |
|
5 days |
|
IV to Oral Switch |
|
Yes, when clinically appropriate |
|
Comments |
|
Antibiotics are not indicated for aspiration without evidence of pneumonia. |
|
Infection |
|
Paediatrics - Community-Acquired Pneumonia: Child < 8 weeks |
|
Likely Organisms |
|
Group B streptococcus, E. coli & other gram negative bacilli, S. aureus, Listeria monocytogenes, CMV, very rarely HSV. |
|
Empiric Antimicrobial Treatment |
|
Recommended antimicrobials as per Paediatrics - Sepsis: Child < 8 weeks |
|
IV to Oral Switch |
|
No, continue IV for entire duration of therapy. |
|
Duration |
| 5 days |
| Comments |
|
Always admit patient to hospital. Stop antibiotics if viral aetiology proven. |
|
Infection |
|
Paediatrics - Community-Acquired Pneumonia: Child > 8 weeks |
|
Likely Organisms |
|
S. pneumoniae, Mycoplasma pneumoniae, H. influenzae, S.aureus, Bordetella pertussis (<3 months), Chlamydia pneumoniae May also be viral: RSV, Parainfluenza |
|
Empiric Antimicrobial Treatment |
|
If well : Amoxicillin PO OR Azithromycin (if patient has already received amoxicillin/co-amoxiclav in the community or presumed atypical infection)
Pneumonia without signs of sepsis or effusion (clinically unwell): Amoxicillin IV (If a sensitive S. aureus is isolated or if pneumatocele, switch to Flucloxacillin IV instead of Amoxicillin) Add Azithromycin PO if
Complicated pneumonia and/or pleural effusion: Cef-UR-oxime IV Plus Azithromycin PO (or Clarithromycin IV if not tolerating PO) If MRSA pneumonia, Add Vancomycin IV ( OR Clindamycin if sensitive) |
|
IV to Oral Switch |
|
Yes, when clinically appropriate. N.B. Cef-UR-oxime PO is not recommended due to low oral bioavailability. Consider cefaclor PO. |
| Duration |
|
Mild to moderate pneumonia: 5 days (3 days for Azithromycin) Complicated pneumonia: 5 - 10 days (3 days for Azithromycin) |
|
Infective Exacerbation of Cystic Fibrosis |
|
Please refer directly to Children's Health Ireland (CHI) 'Clinibee' Antimicrobial Guidelines app for guidance. Also, contact Microbiology for advice if needed. |
|
Infection |
|
Paediatrics - Pertussis (Whooping Cough) |
|
Likely Organisms |
|
Bordetella pertussis |
|
Empiric Antimicrobial Treatment |
|
Azithromycin PO (unlicensed for pertussis): Age < 6 months: 10mg/kg once daily for 3 days. Age > 6 months: 10mg/kg (max 500mg) once daily for 3 days. |
|
Duration of Therapy |
|
3 days |
|
Comments |
|
Chemoprophylaxis for household contacts: As directed by Public Health. Azithromycin PO prophylaxis dose is the same as treatment dose for pertussis. References for azithromycin dosing for pertussis:
|
|
Infection |
|
Paediatrics - Pneumonia in hospital inpatients with or without severe infection (HDU/PICU) or risk factors:
|
|
Consult Microbiology |
Paediatrics - Sepsis
|
Infection |
|
Paediatrics - Sepsis: Child < 8 weeks Excludes neutropenic sepsis |
|
Likely Organisms |
|
Child < 8 weeks (chronological age) Group B Streptococcus, E. coli, Listeria monocytogenes, N. meningitidis, S. pneumoniae |
|
Empiric Antimicrobial Treatment |
|
For pre-term infants or previous NICU admission, refer patient to Neonatology / Microbiology. Child < 8 weeks (chronological age) Cef-O-taxime IV Plus Amoxicillin IV Plus consider (see comments below): +/- Gentamicin IV +/- Vancomycin IV +/- Aciclovir IV Plus contact Microbiology if recent foreign travel for mother or baby in case of potential for colonisation with resistant organism.
Add Gentamicin if : • Severe sepsis/ haemodynamically unstable • Requiring inotropes/critical care • Likely resistant organisms e.g., frequent or prolonged hospitalisation; >48 hours following admission; recent foreign travel for mother or baby.
Add Vancomycin if: • MRSA positive • Recent travel outside of Ireland for mother or baby • Prolonged antibiotics in past 3 months • Concern about infected prosthetic material e.g. PICC line in-situ.
Add Aciclovir if clinical features of HSV.
Add Clindamycin if suspected staphylococcal/streptococcal toxic shock.
If suspected abdominal source, please see monograph for Paediatric Intra-Abdominal Infections . |
|
Duration of Treatment |
|
Duration depends on source of sepsis. If cultures are negative and sepsis is not suspected, discontinue antibiotics. |
|
Comments |
|
|
Infection |
|
Paediatrics - Sepsis: Child > 8 weeks Excludes neutropenic sepsis |
|
Likely Organisms |
|
Child > 8 weeks N. meningitidis, S. pneumoniae, H. Influenzae, Group B Streptococcus if ≤12 weeks |
|
Empiric Antimicrobial Treatment |
|
Child > 8 weeks Cef-O-taxime IV Plus consider (see comments below): +/- Gentamicin IV +/- Vancomycin IV +/- Aciclovir IV Plus contact Microbiology if recent foreign travel for mother or baby in case of potential colonisation with resistant organism.
Add Gentamicin if: • Severe sepsis/ haemodynamically unstable • Requiring inotropes/critical care • Likely resistant organisms e.g., frequent or prolonged hospitalisation; >48 hours following admission; recent foreign travel for mother or baby.
Add Vancomycin if: • MRSA positive • Recent travel outside of Ireland for mother or baby • Prolonged antibiotics in past 3 months • Concern about infected prosthetic material e.g. PICC line in-situ.
Add Aciclovir if clinical features of HSV.
Add Clindamycin if suspected staphylococcal/streptococcal toxic shock.
If suspected abdominal source, please see monograph for Paediatric Intra-Abdominal Infections . |
|
Duration of Treatment |
|
Duration depends on source of sepsis. If cultures are negative and sepsis is not suspected, discontinue antibiotics. |
|
Comments |
|
Paediatrics - Skin, Soft Tissue and Surgical Wound Infections
|
Infection |
|
Paediatrics - Animal or Human Bites |
|
Likely Organisms |
|
Pasteurella species, oral anaerobes, S. aureus, beta haemolytic streptococci |
|
Empiric Antimicrobial Prophylaxis |
|
1. No prophylaxis offered if skin is unbroken 2. Consider prophylaxis if skin is broken but not drawn blood if:
3. Offer antibiotic prophylaxis if skin is broken and drawn blood 4. Prescribe Co-amoxiclav PO |
|
Empiric Antimicrobial Treatment of Infected Bite |
|
Co-amoxiclav PO
If penicillin allergic: Co-trimoxazole PO |
| Duration |
|
Prophylaxis: 3 days Treatment of infected bite: 5 days. |
| Comments |
|
Assess the risk of tetanus, rabies or a bloodborne viral infection and take appropriate action. Ask about tetanus immunisation status. Manage the wound with irrigation and debridement as necessary. Other animal bites discuss with ID / Micro. |
|
Infection |
|
Paediatrics - Burns |
|
Likely Organisms |
|
Group A Streptococcus, S. aureus If infection occurs > 5 days post-hospitalisation, also aerobic gram negative organisms (e.g.Pseudomonas aeruginosa) |
|
Empiric Antimicrobial Treatment |
|
Burns should not initially be treated with antibiotics. Treat only if infected and on the advice of the Consultant . Be aware of potential for toxic shock syndrome in children who often have only relatively small burns (fever, rash, diarrhoea, and ultimately shock).
Flucloxacillin IV OR Cef-AZ-olin IV 25mg/kg TDS (Max 6g/day) Plus if severe infection: Clindamycin PO/IV Plus if infection occurs > 5 days post-hospitalisation: Pip/tazobactam IV |
|
Duration of Treatment |
|
7 days, including IV to oral switch if appropriate oral option available based on C&S. |
|
IV to Oral Switch |
|
Yes, when clinically appropriate |
| Comments |
| Ensure appropriate swabs are sent. Aim to rationalise based on sensitivities. |
|
Infection |
|
Paediatrics - Cellulitis or Erysipelas |
|
Likely Organisms |
|
Group A Streptococcus, S. aureus |
|
Empiric Antimicrobial Treatment |
|
Mild to Moderate: Flucloxcillin PO OR Cef-AL-exin PO
Severe: Cef-AZ-olin IV 25mg/kg TDS (Max 6g/day) OR Flucloxacillin IV Plus Clindamycin PO/IV |
|
Duration of Treatment |
|
5 to 7 days, including IV to oral switch. If infection near eyes or nose: 7 days. Full resolution after 5-7 days is not expected as skin takes time to return to normal. Courses can be extended (up to 14 days in total) if necessary, based on clinical assessment. |
|
IV to Oral Switch |
|
Yes, when clinically appropriate |
|
Comments |
|
In severe cellulitis, seek urgent Microbiology consult . |
|
Infection |
|
Paediatrics - Cellulitis post varicella infection |
|
Likely Organisms |
|
Group A Streptococcus, S. aureus |
|
Empiric Antimicrobial Treatment |
|
Cef-O-taxime IV Plus Clindamycin PO/IV If MRSA, Add Vancomycin IV |
|
Duration of Treatment |
|
Consult Microbiology |
|
IV to Oral Switch |
|
Yes, when clinically appropriate |
|
Infection |
|
Paediatrics - Impetigo |
|
Likely Organisms |
|
Group A Streptococcus, S. aureus |
|
Empiric Antimicrobial Treatment |
|
Mild or localised infection: Topical fusidic acid cream Widespread or recurrent infection: Flucloxacillin PO OR Cef-AL-exin PO |
|
Duration of Treatment |
|
5 to 7 days. |
|
Infection |
|
Paediatrics - Severe Skin and Soft Tissue Infection with Systemic Illness: e.g. Necrotising fasciitis, Toxic shock-like illness |
|
Likely Organisms |
|
Group A Streptococcus, S. aureus |
|
Empiric Antimicrobial Treatment |
|
SEEK URGENT ADVICE FROM MICROBIOLOGY. |
|
Duration of Treatment |
|
Consult Microbiology. |
|
IV to Oral Switch |
|
Consult Microbiology. |
|
Comments |
|
Seek advice regarding need for surgical debridement. Consider IVIG if signs suggest toxic shock syndrome. Broader antibiotic cover given initially until clear microbiologic diagnosis established, then adjust antimicrobials to culture and sensitivity results. |
|
Infection |
|
Paediatrics - Surgical Site Infection |
|
Likely Organisms |
|
S. aureus predominantly |
|
Empiric Antimicrobial Treatment |
|
Mild: Flucloxacillin PO OR Cef-AL-exin PO
Moderate to severe: Flucloxacillin IV OR Cef-AZ-olin IV 25mg/kgTDS (max 6g/day)
If contaminated wound or slow to respond, Gram negative organism suspected, Add Piperacillin/tazobactam IV. If known MRSA, consult Microbiology. |
|
Duration of Treatment |
|
5 days. |
|
Comments |
|
Review empiric antimicrobials based on culture and sensitivity results. |
Paediatrics - Urinary Tract Infections
The following advice pertains to a child who has had a single UTI only. If previous or recurrent UTIs, please check previous antimicrobial susceptibilities.
|
Infection |
|
Paediatrics - UTI: Child < 2 months old |
|
Likely Organisms |
|
E. coli, Proteus species, Klebsiella, other aerobic gram negative bacilli, enterococci |
|
Empiric Antimicrobial Treatment |
|
Amoxicillin IV Plus Cef-O-taxime IV Plus consider (see comments below): +/- Gentamicin IV Plus contact Microbiology if recent foreign travel for mother or baby in case of potential for colonisation with resistant organism.
Add Gentamicin if : • severe sepsis/haemodynamically unstable •requiring inotropes/ critical care •l ikely resistant organisms e.g., frequent or prolonged hospitalisation; >48 hours following admission; recent foreign travel for mother or baby.
|
|
Duration of Treatment |
|
10 days |
|
IV to Oral Switch |
|
Age dependent. |
|
Comments |
|
|
Infection |
|
Paediatrics - UTI: Child > 2 to 6 months old |
|
Likely Organisms |
|
E. coli, Proteus species, other aerobic gram negative bacilli, enterococci |
|
Cef-UR-oxime IV +/- Gentamicin IV |
|
Duration of Treatment |
|
10 days total including IV to PO switch |
|
IV to Oral Switch |
|
N.B. Cef-UR-oxime PO is not recommended due to low oral bioavailability. Choice of PO antibiotic to be based on C&S. Children can be switched to oral antibiotics and sent home after 48 hours if:
|
|
Infection |
|
Paediatrics - UTI: Child > 6 months |
|
Likely Organisms |
|
E. coli, Proteus species, other aerobic gram negative bacilli, enterococci |
|
Empiric Antimicrobial Treatment |
|
If systemically unwell: Cef-UR-oxime IV +/- Gentamicin IV Lower UTI and well: Cef-AL-exin PO or Trimethoprim PO or Nitrofurantoin PO# # N.B. Nitrofurantoin liquid is unlicensed and not readily available in the community. Contact community pharmacy prior to discharge. |
|
Duration of Treatment |
|
If systemically unwell: 7 to 10 days total including IV to PO switch Lower UTI and well: 3 days |
|
IV to Oral Switch |
|
N.B. Cef-UR-oxime PO is not recommended due to low oral bioavailability. Choice of PO antibiotic to be based on C&S. Children can be switched to oral antibiotics and sent home after 48 hours if:
|
Paediatric Gentamicin Once Daily Guideline

Children's Health Ireland Paediatric Gentamicin Dosing and Monitoring Guideline 2020
EXCEPTIONS to this guide
- This guideline does not apply to those with known mitochondrial m.1555A>G mutation.
- This guideline excludes patients on renal replacement therapy; consult local protocols and nephrology department for advice in these cases.
PAEDIATRIC GENTAMICIN DOSING GENERAL GUIDANCE
- Use extended interval or once daily dosing except where recommended by ID/Micro.
- Dose using ideal weight for height in obesity (dose should not exceed maximum adult daily dose of 480mg or as per local policy). However, individual hospitals may have specific protocols for particular patient groups which recommend different maximum daily doses.
- Dose based on kidney function (i.e. review urea and creatinine, urine output, consider any known kidney abnormality or dialysis. However, this should not delay the first dose of gentamicin in patients with suspected sepsis).
- If possible, dehydration should be corrected before starting gentamicin.
- Assess need to continue other ototoxic or nephrotoxic drugs. Where concomitant use is unavoidable, administration should be separated by as long a period as practicable (e.g. gentamicin and an ototoxic diuretic such as furosemide).
- Regular review and documentation of ongoing need for gentamicin is essential.
- The time of blood sampling and the time the last dose was administered must be recorded in order to accurately interpret gentamicin level results.
- This guideline excludes patients on renal replacement therapy; consult local protocols and nephrology department for advice in these cases.
PAEDIATRIC GENTAMICIN INITIAL DOSING REGIMEN
NOTE: Dosing guidelines in individual centres should be agreed locally with input from microbiology/ infectious disease experts, nephrologists and pharmacy.
|
Age |
Renal Function |
Initial Dose |
|
Child > 1 month |
Normal |
7mg/kg 24 hourly IV infusion over 30 min |
|
Updated Renal Dosing from Crumlin/Temple St Hospitals 2019: |
||
|
Child > 1 month |
Mild renal impairment (GFR 30 – 70ml/min/1.73 m 2 ) |
5mg/kg, prescribe single dose only |
|
Child > 1 month |
Moderate renal impairment (GFR 10 – 30 ml/min/1.73 m 2 ) |
3mg/kg, prescribe single dose only |
|
Child > 1 month |
Severe renal impairment (HD/GFR < 10 ml/min/1.73 m 2 ) |
2mg/kg, prescribe single dose only |
PAEDIATRIC GENTAMICIN MONITORING
Why are levels taken?
- Pre dose (trough) levels are taken to ensure that the previous dose of gentamicin has been sufficiently cleared by the kidneys before the next dose is given. Failing to clear doses due to kidney impairment can result in toxic levels and kidney damage.
- Post dose (peak) levels are not routinely performed with extended interval or once daily dosing. They may occasionally be required, but should only be done under expert guidance.
When should first level be taken?
- The prescriber must decide on initial timing of therapeutic drug monitoring (TDM) and order first serum pre-dose level in advance of prescribing 1st dose if more than one dose is planned.
- The first pre dose level can be taken either before the 2nd or the 3rd dose depending on the clinical situation.
-
For the majority of patients with normal kidney function, taking a pre-dose level before the 3rd dose is appropriate. This prevents unnecessary levels from being taken in patients that are likely to stop gentamicin within the first 36-48 hours of therapy. For example:
- Patients who are likely to be switched to oral antibiotics after 48 hours of IV therapy e.g. treatment of uncomplicated UTI.
- Patients being treated for febrile neutropenia who are well, with no clinical focus of infection and where gentamicin will be stopped after 48 hour negative cultures.
-
If there are any concerns about a patient’s kidney function a level should be taken before the 2nd dose of gentamicin. For example:
- Patients with acute kidney impairment due to sepsis/or with profound circulatory compromise and/or on inotropes especially in intensive care settings.
- Any patient with chronic kidney impairment or with a known kidney abnormality.
Timing of levels
- Ideally the blood sample should be taken immediately before the next dose is due.
-
However in order to facilitate phlebotomy and laboratory times, levels can be taken in the following time windows:
- Up to 8 hours before dose is due if on 24 hourly dosing (i.e. 16-24 hours post dose)
- Up to 8 hours before dose is due if on 36 hourly dosing (i.e. 28-36 hours post dose)
- The time of blood sampling and the time previous dose was administered must be recorded in order to accurately interpret the results.
Subsequent doses
- Give 2nd or 3rd dose as appropriate without waiting for result, unless there is evidence of kidney dysfunction (e.g. elevated serum creatinine or urea concentrations, decreased urine output).
- In patients with kidney dysfunction, wait for result before giving any further doses.
- In acutely septic patients, dose may be given if clinically appropriate under direction of a senior clinician.
Interpreting results
Aim for pre-dose levels < 1mg/L for paediatric patients. If levels are above recommended range:
- Double check that the level was taken in the correct time window (i.e.16-24 hours or 28-36 hours post dose as appropriate).
- If the levels are high in acutely septic patients, contact ID team/Microbiology Consultant for advice.
- In patients with a level >1mg/L who are not acutely septic, hold the next dose and repeat level 12 hours later.
- Recommence dosing if levels are ≤1mg/L and amend the dosage interval to reflect the time required to clear the previous dose (e.g. from every 24 hours to every 36 hours).
Frequency of monitoring
- Check U&E/creatinine each time you check gentamicin level
- In patients with normal kidney function: Repeat level every 3 doses.
- In patients with kidney impairment: Before every dose until discussed with Consultant Nephrologist/Microbiologist or ID.
- More frequent monitoring may be required if the patient is on concomitant nephrotoxic drugs (e.g. ibuprofen, ciclosporin, tacrolimus, furosemide, ACE inhibitors), if the dose has changed or kidney function deteriorates.
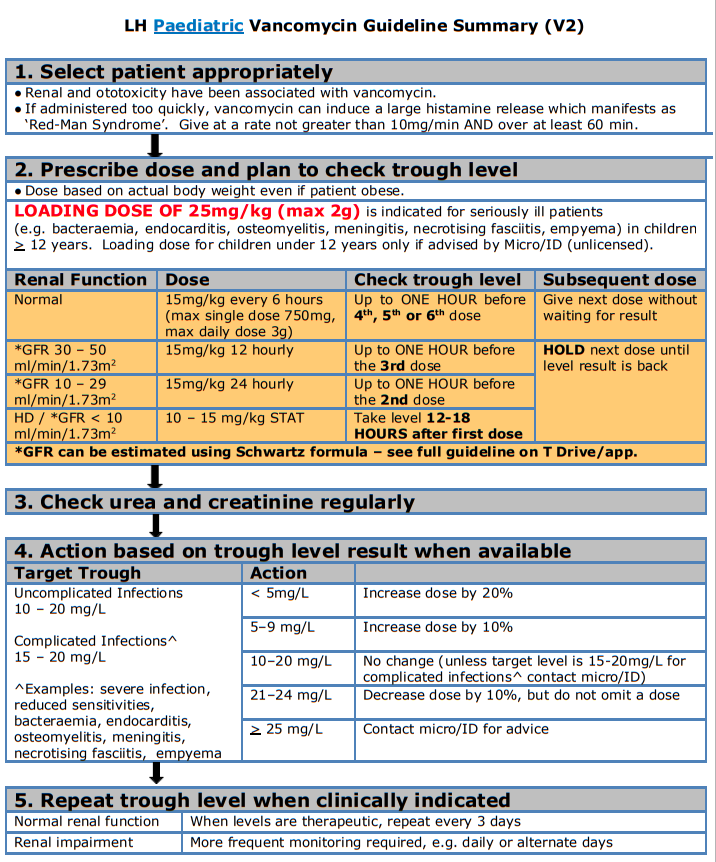
Paediatric Vancomycin Guideline
Children's Health Ireland Vancomycin Paediatric Dosing and Monitoring Guideline 2020
Background
Vancomycin is a glycopeptide antibiotic active against Staphylococcus aureus and other gram positive susceptible bacterial infections. It is indicated for use when there is resistance pattern such as methicillin-resistant Staphylococcus aureus (MRSA) or when the patient demonstrates intolerance to alternative antibiotics. It is not the first line treatment for methicillin-sensitive Staphylococcus aureus (MSSA) as it is less effective than beta-lactams.
- Mechanism of action: Vancomycin acts by inhibiting the production of the peptidoglycan polymers of the bacterial cell wall by preventing the transfer and addition of the muramylpentapeptide building blocks that make up the peptidoglycan molecule itself.
- Time dependent killing: For vancomycin, it has been shown that its efficacy is best predicted by the area under the concentration-time curve over 24 hours (AUC24) divided by the MIC (AUC/MIC). This method of therapeutic drug monitoring is not practical at a ward level, therefore trough levels taken an hour before the dose is due is recommended in this guideline to determine efficacy.
- When treating an infection caused by bacteria with a vancomycin MIC less than 1 mg/L, aim for a trough of 10-15mg/L
- If the vancomycin MIC is greater than 1 mg/L, a trough of 15-20mg/L may be required
Under dosing and sub-therapeutic levels may result in the emergence of drug resistance and subsequent treatment failure.
Adverse Effects
- Common: Decrease in blood pressure, flushing of the upper body (“red man syndrome”), exanthema and mucosal inflammation, pruritus, urticarial, renal insufficiency, increased serum creatinine and urea.
- Uncommon: Transient or permanent loss of hearing (ototoxicity associated with persistent high levels).
- Rare: Hypersensitivity reactions, anaphylactic reactions, vertigo, tinnitus, dizziness, nausea.
- Rapid infusions can result in “Red man syndrome”. “Red man” is a red rash over the upper body that is mediated by a mass histamine release. It is NOT an allergy - please contact Micro/ID for advice. Note the rate and the concentration the rate reaction has occurred. Further infusions should be run at a slower rate and more dilute concentration. Document changes in the drug kardex and patient notes.
Vancomycin Dosing, Therapeutic Drug Monitoring and Dose Adjustments in Patients with Normal Renal Function
A. Vancomycin Dosing in Normal Renal Function
For child > 1 month with normal renal function:
- Vancomycin 15mg/kg 6 hourly IV (Maximum single dose 750mg, maximum daily dose 3g)
- Loading dose of 25mg/kg (max 2g) can be given to achieve faster therapeutic levels. A loading dose would be indicated for patients with bacteraemia, endocarditis, osteomyelitis, meningitis, necrotising fasciitis and empyema for children 12 years and above or under if advised by Micro/ID.
B. Vancomycin Monitoring in Normal Renal Function
- Levels should be taken through venepuncture/capillary blood samples.
- Do not withhold the dose when waiting for a level to come back.
- Sub-therapeutic levels can result in treatment failure or the emergence of drug resistance.
- Toxic or high levels of vancomycin can result in nephrotoxic and/or ototoxicity. It is important to monitor renal function for the duration of treatment of vancomycin as it is renally cleared. Monitor creatinine and urea a minimum of twice weekly for the duration of vancomycin treatment.
- If a prolonged course of vancomycin is required a base line auditory test should be carried out.
- If the patient is on additional nephrotoxic medication (e.g. NSAIDs, aciclovir, aminoglycosides, diuretics, omeprazole), monitor renal function more frequently. For Acute Kidney Injury (AKI) monitoring and classification please see section on renal impairment below.
- NB: Check U&E/ creatinine each time you check a vancomycin level.
|
Important Vancomycin levels are processed in the Biochemistry Laboratory in OLOL from 8am to 8pm Mon - Fri and from 9am to 5pm on Sat - Sun. Consultant request only outside of these hours. |
|
Type of Infection |
Target trough concentration |
|
|
Uncomplicated infections |
10 to 20 mg/L |
|
|
Complicated infections (e.g. bacteraemia, endocarditis, osteomyelitis, meningitis, necrotising fasciitis and empyema) |
15 to 20 mg/L |
|
|
Dosing Frequency |
When to take trough level |
|
|
Six hourly |
Up to ONE HOUR before 4 th , 5 th or 6 th dose When levels are therapeutic, repeat every 3 days |
|
|
In patients with normal renal function, DO NOT withhold the next dose while awaiting the result of the trough level – this may result in the patient being under dosed |
||
C. Vancomycin Recommended Dose Adjustment based on Trough Level Results
- After all dose adjustments repeat level as per recommendations above
- If there is a rise in creatinine, please calculate GFR and dose adjust as per recommendations in section on renal impairment below. Additionally assess patient for AKI as per the KDIGO definition in section on renal impairment below.
|
Trough level interpretation and maintenance dose adjustment for child >1 month (15mg/kg IV 6 HOURLY) |
||
|
Target trough level |
Trough level |
Dose Adjustment |
|
10 – 20 mg/L If signs of AKI contact micro/ID and see section on renal impairment below |
< 5mg/L |
Increase dose by 20% |
|
5–9 mg/L |
Increase dose by 10% |
|
|
10–20 mg/L |
No change (unless target level is 15-20mg/L for complex infections* contact micro/ID) |
|
|
21–24 mg/L |
Decrease dose by 10%, but do not omit a dose |
|
|
> 25 mg/L |
Contact micro/ID for advice |
|
|
*Complicated infections: Severe infection, reduced sensitivities, bacteraemia, endocarditis, osteomyelitis, meningitis, necrotising fasciitis and empyema |
||
Vancomycin Dosing, Therapeutic Drug Monitoring and Dose Adjustments in Patients with Renal Impairment
- Dosing is based on estimated GFR in patients with renal impairment. Please use the Schwartz formula below to calculate GFR
- Dosing and monitoring are expressed in the table below
- Please be aware that impaired renal function should be taken into account for both chronic kidney disease (CKD) and in acute kidney injury (AKI)
- If trough is high, consult nephrologist/Micro/ID for advice on subsequent dosing
|
GFR can be estimated by the Schwartz formula: Child over 1 year: GFR (mL/min/1.73 m 2 ) = (40 × Height in cm) / Creatinine in micromol/L Neonate: GFR (mL/min/1.73 m 2 ) = (30 × Height in cm) / Creatinine in micromol/L |
To monitor for AKI please use the KDIGO model, if a patient is showing signs of AKI please review vancomycin and all nephrotoxic medication prescribed.
|
KDIGO classification of Acute Kidney Injury (AKI) Stage 1 : Increase in creatinine of ≥50% Or Absolute increase in creatinine of 26.5micromol/L Stage 2 : Increase in creatinine of ≥100% Stage 3 : Increase in creatinine of ≥200% Reference: Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2012;2:8. |
|
Dose Adjustment and Monitoring for Renal Impairment in Infants and Children >1 month of age |
|||
|
GFR (mL/min/1.73m 2 ) |
IV Dose |
Frequency |
When to take a trough level |
|
30-50 |
15mg/kg |
12 hourly |
Up to ONE HOUR before the 3rd dose . HOLD the next dose until level is back. |
|
10-29 |
15mg/kg |
24 hourly |
Up to ONE HOUR before the 2nd dose . HOLD the next dose until level is back. |
|
<10 / HD / PD |
10 – 15mg/kg |
STAT – subsequent dosing determined by serum levels. |
Take level 12-18 HOURS after first dose . HOLD the next dose until level is back. |
Vancomycin Reconstitution and Administration
|
Dilution of reconstituted vials (500mg and 1g) |
Dilute with sodium chloride 0.9% or glucose 5% to a concentration of up to 5mg/mL i.e. dilute each 500mg with at least 100mL |
|
Rate of infusion |
The rate must not exceed 10mg/minute, give over at least 60 minutes minimum using an infusion pump e.g. 750mg over at least 75 minutes, 1000mg over at least 100 minutes, etc |
|
Infusion reactions |
Rapid infusion may cause severe hypotension (including shock and cardiac arrest), wheezing, dyspnoea, urticaria, pruritus, flushing of the upper body (‘red man' syndrome), pain and muscle spasm of back and chest. Stop the infusion if they occur. Effects may last between 20 minutes and up to several hours after stopping administration. Peripheral administration may cause injection site pain and thrombophlebitis - rotate injection sites. |