Neonatal Guidelines
Neonatal Antimicrobial IV Monographs
Neonatal - Aciclovir IV
Neonatal - Aciclovir IV
Antiviral [1].
MEDICATION SAFETY ISSUES
- Aciclovir may be confused with other antivirals [2]
- Zovirax® may be confused with Zyvox® [2,3]
- Anaphylaxis has been reported very rarely with aciclovir [4].
- Zovirax® may be confused with Zithromax® [2].
USES
- Treatment of herpes simplex and varicella zoster infections [1].
- Under consultant neonatologist and virologist direction, prophylactic IV aciclovir should be considered for neonates whose mothers develop chickenpox 7 days before to 7 days after delivery due to the risk of severe complicated infection despite varicella-zoster immunoglobulin prophylaxis [5].
PRESENTATION
Concentrate for solution for infusion: Aciclovir 250mg/10ml [4].
DOSAGE [1]
|
Age |
Infection |
Dose |
Frequency |
Duration |
|
Neonate |
Prophylaxis of chickenpox after delivery |
10mg/kg |
Every 8 hours |
Until blood test confirms absence of virus |
|
Neonate and Child 1 – 3 months |
Herpes simplex |
20mg/kg |
Every 8 hours |
14 days (for at least 21 days if CNS involvement, confirm CSF negative for HSV before stopping treatment) |
|
Herpes zoster |
10 – 20 mg/kg |
Every 8 hours |
At least 7 days (for 10 – 14 days in encephalitis, possibly longer if also immunocompromised) |
Renal Impairment : Dose reduction may be required – refer to BNFc and seek advice [1]. Nephrotoxic drugs (e.g. gentamicin, vancomycin) may worsen renal function if used concurrently.
RECONSTITUTION [1,4,6].
In OLOL, aciclovir is available as 250mg/10ml concentrated solution. The vial must be further diluted before use. Dilute 4ml of the 25mg/ml solution with 16 ml Sodium Chloride 0.9% to a final volume of 20ml.
The resulting solution contains aciclovir 5mg/ml.
ADMINISTRATION
Administer by IV infusion over 1 hour [1,4].
SAMPLE CALCULATION
3.2kg neonate 7 days old with herpes simplex infection. Dose 20mg/kg = 64mg every 8 hours.
Reconstitute aciclovir as above to a solution containing 5mg/ml.
Administer 12.8ml (64mg) by IV infusion over 1 hour.
STORAGE
Store unopened vials at room temperature below 25 o C. Once diluted, use immediately. Discard any remaining solution [4].
MONITORING
- Maintain adequate hydration, especially with infusion of high doses, or during renal impairment [1,4]
- Monitor for changes in renal function [7], especially when administered concomitantly with nephrotoxic drugs (eg. gentamicin, vancomycin)
- Patients with renal impairment are at increased risk of developing neurological side effects and should be closely monitored for evidence of these effects. Dose reduction is required in patients with renal impairment [1,4].
- Monitor liver function [2].
- Monitor infusion site for phlebitis if administered peripherally [6]
- Monitor neutrophil count at least twice weekly in neonates receiving aciclovir 60 mg/kg/day IV for more than 48 hours [2].
ADVERSE EFFECTS
Nausea, vomiting, abdominal pain, diarrhoea, headache, fatigue, rash, urticaria, pruritus, photosensitivity; very rarely hepatitis, jaundice, dyspnoea, neurological reactions (including dizziness, confusion, hallucinations, convulsions, ataxia, dysarthria, and drowsiness), acute renal failure, anaemia, thrombocytopenia, and leucopenia; on intravenous infusion , severe local inflammation (sometimes leading to ulceration), and very rarely agitation, tremors, psychosis and fever [1].
REFERENCES
- British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health, et al. BNF for Children. London: BMJ Group, Pharmaceutical Press and RCPCH Publications Limited; 2020. Available from www.medicinescomplete.com , accessed 16/11/2020.
- UpToDate. Acyclovir Paediatric Drug Information: Lexicomp®. Available from www.uptodate.com , accessed 16/11/2020..
- Institute for Safe Medication Practices. ISMP’s List of Confused Drug Names, 2019. Available from www.ismp.org, accessed 16/11/2020.
- Pfizer Healthcare Ireland. Summary of Product Characteristics for Aciclovir 25mg/ml Concentrate for Solution for Infusion. 2018. Available from www.hpra.ie , accessed 16/11/2020.
- Royal College of Obstetricians and Gynaecologists. Chickenpox in Pregnancy. Green top Guideline No. 13. January 2015.
- Medusa Injectable Drugs Guide. Aciclovir Intravenous Infusion (concentrated solution) – Paediatric Monograph, 2018. Available from www.medusa.wales.nhs.uk , accessed 16/11/2020.
- Department of Health and Government of South Australia, South Australian Neonatal Medication Guidelines - Aciclovir 2017. Available from http://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/clinical+resources/clinical+topics/neonatal+medication+guidelines , accessed 16/11/2020.
Summary of Changes from Previous Versions
|
Date |
Change |
|
Feb 2021: Rev. No. 2 |
Updated based on Rotunda Aciclovir Monograph Jan 2019. OLOL changes:
|
|
Aug 2017: Rev. No. 1 |
Aciclovir Claris® solution for infusion now stocked in OLOL, therefore no need to reconstitute power for solution for infusion. |
|
May 2015 |
This is the first version of this guideline. |
Neonatal - Ambisome IV
Neonatal - Ambisome® IV (Liposomal Amphotericin B)
NB. Prescribe by BRAND name only.
Lipid formulation of the antifungal amphotericin B - significantly less toxic than the conventional form [1].
MEDICATION SAFETY ISSUES
- Amphotericin is included on the Institute for Safe Medication Practices (ISMP) List of High-Alert Medications [2]. Mix-ups between different formulations have led to overdoses (sometimes fatal) or underdoses resulting in subtherapeutic treatment [2-4].
- There are two liposomal amphotericin B products available, Ambisome® and Abelcet®. A conventional formulation is also available - Fungizone®. NB. These preparations are not interchangeable . Prescribe Ambisome® by brand name only to avoid confusion . [1,5]
USES
First-line antifungal for invasive infections [1]. Not licensed for use in infants under one month [1,6].
PRESENTATION
Ambisome® 50mg Powder for Concentrate for Dispersion for Infusion [6]
DOSAGE [1]
|
Age |
Dose |
Frequency |
|
Neonate and Child 1 – 3 months |
3mg/kg
|
Every 24 hours |
On advice from the Consultant Microbiologist, the dose may be increased to 5mg/kg if required.
RECONSTITUTION [1,6-9]
- Check that the prescription specifies Ambisome® and the product you are using is Ambisome®.
- Ambisome® must be reconstituted with sterile Water for Injection (WFI) and diluted in Glucose 5% ONLY. AMBISOME® IS INCOMPATIBLE WITH SODIUM CHLORIDE 0.9%.
- NB. There are TWO STEPS for reconstitution. BOTH Step 1 and Step 2 below must be followed.
- Step 1 : Add 12ml WFI to the 50mg Ambisome® vial and shake vigorously for 30 seconds to completely disperse the drug. The resulting solution contains 4mg/ml.
- Step 2 : Using the 5 micron filter provided, further dilute 4ml of this solution with 12ml of Glucose 5% to a final volume of 16ml. The resulting solution contains 1mg/ml Ambisome®.
- If the baby is fluid restricted, Ambisome® may be reconstituted to a concentration of 2mg/ml instead.
Contact the Pharmacy Department to order subsequent doses of Ambisome® 1mg/ml reconstituted solution (10ml volume) from external compounding unit if available.
ADMINISTRATION [1,6,10]
- AMBISOME® IS INCOMPATIBLE WITH SODIUM CHLORIDE 0.9%.
- Prime the IV line (T-piece) with Glucose 5% before and after administration (or use a separate line).
- Administer by IV infusion - an in-line membrane filter (not less than 1 micron) may be used.
- Administer by IV infusion over 1 hour.
- Monitor vital signs and observe patient for infusion-related reactions.
SAMPLE CALCULATION
3.2kg neonate 9 days old with invasive candida infection. Dose: 3mg/kg = 9.6mg every 24 hours.
Reconstitute and further dilute Ambisome® as above to a solution containing 1mg/ml.
Required dose 9.6mg = 9.6ml. Administer by IV infusion over 1 hour.
STORAGE
Store unopened vials at room temperature below 25 o C. Once reconstituted, use immediately. [6].
MONITORING [1,7,8]
- Observe patient for infusion reactions during each infusion – a slower rate may reduce reactions.
- Caution if concomitant administration of other nephrotoxic agents.
- Monitor renal function and serum magnesium daily initially, then two to three times weekly
-
Monitor liver function and FBC twice weekly
- Hypokalaemia is common
- Hypomagnesaemia is common and may require supplementation
- Hyponatraemia may occur - ensure adequate hydration.
ADVERSE EFFECTS
Fever and chills/rigors are the most frequent infusion-related reactions [6]. Less frequent infusion-related reactions include chest tightness or pain, dyspnoea, bronchospasm, flushing, tachycardia, hypotension, and musculoskeletal pain. [6]. Other adverse effects include nausea, vomiting, abdominal pain, diarrhoea, cardiovascular effects (including arrhythmias), headache, febrile reactions, electrolyte disturbances (including hypokalaemia and hypomagnesaemia), disturbances in renal function (including renal tubular acidosis), abnormal liver function (discontinue treatment), blood disorders (including anaemia, thrombocytopenia), rash; less commonly neurological disorders (including convulsions, peripheral neuropathy, tremor, encephalopathy, hearing loss, diplopia); also reported anorexia, myalgia, arthralgia, toxic epidermal necrolysis, Stevens-Johnson syndrome [1].
REFERENCES
- British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health, et al. BNF for Children. London: BMJ Group, Pharmaceutical Press and RCPCH Publications Limited; 2020. Available from www.medicinescomplete.com , accessed 16/11/2020.
- Institute for Safe Medication Practices. ISMP List of High-Alert Medications in Acute Care Settings. 2018. Available from www.ismp.org , accessed 16/11/2020.
- Institute for Safe Medication Practices (ISMP). Worth Repeating... Preventing mix-ups between various formulations of amphotericin B. ISMP Medication Safety Alert! , September 6, 2007 . Available from www.ismp.org , accessed 09/12/14.
- National Patient Safety Agency (NPSA). Risk of confusion between non-lipid and lipid formulations of injectable amphotericin. Rapid Response Report 2. September 3, 2007. DH Gateway Reference 8726. Available from www.npsa.nhs.uk , accessed 09/12/14.
- Institute for Safe Medication Practices. ISMP’s List of Confused Drug Names, 2019. Available from www.ismp.org , accessed 16/11/2020.
- Gilead Sciences International Limited. Summary of Product Characteristics for AmBisome Liposomal Amphotericin B 50mg Powder for Concentrate for Dispersion for Infusion. 2018. Available from www.hpra.ie , accessed 16/11/2020.
- Department of Health and Government of South Australia, South Australian Neonatal Medication Guidelines – Amphotericin (liposomal) 2018. Available from http://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/clinical+resources/clinical+topics/neonatal+medication+guidelines , accessed 16/11/2020.
- Injectable Drugs Guide. Available from www.medicinescomplete.com , accessed 16/11/2020.
- Institute for Safe Medication Practices (ISMP) and Vermont Oxford Network (VON). Standard concentrations of neonatal drug infusions. 2011. Available from www.ismp.org , accessed 09/12/14.
- Guys and St Thomas Paediatric Formulary 2012. Available online, on file in OLOL Pharmacy Department.
Summary of Changes from Previous Versions
|
Date |
Change |
|
Feb 2021: Rev. No. 1 |
Updated based on Rotunda Ambisome® Monograph Jan 2019. OLOL changes: References updated. |
|
August 2015 |
This is the first version of this guideline. |
Neonatal - Amoxicillin IV and PO
Neonatal - Amoxicillin IV and PO
Amoxicillin is a broad-spectrum penicillin antibiotic which is rapidly bactericidal against certain Gram-positive and Gram-negative organisms [2].
MEDICATION SAFETY ISSUES
- Anaphylaxis and other hypersensitivity reactions have been reported [1].
- Sound alike drugs: Amoxil® may be confused with Augmentin® [3]
USES
Amoxicillin is indicated for the treatment of commonly occurring bacterial infections, in particular it is used to treat listeria and enterococcal infections which are cephalosporin resistant [2].
PRESENTATION
Intravenous: Powder for Solution for injection or infusion: Amoxil® vials 500mg [2]. Generic brands will be supplied when Amoxil® is in short supply.
Oral: Powder for oral suspension Amoxicillin 125mg/5mL [6].
DOSAGE [1]
For susceptible infections including urinary-tract infections, sinusitis; Haemophilus influenzae infections, Listerial meningitis, Group B streptococcal infection, enterococcal endocarditis (in combination with other antibiotics)
Intravenous Infusion
|
Age |
Dose |
Frequency |
Max Dose |
|
Neonate under 7 days |
50mg/kg |
Every 12 hours |
Dose doubled in meningitis |
|
Neonate 7-28 days |
50mg/kg |
Every 8 hours |
Dose doubled in meningitis |
|
Child 1 to 3 months |
50mg/kg |
Every 4-6 hours |
Max 2g every 4 hours |
Oral
|
Age |
Dose |
Frequency |
Max Dose |
|
Neonate under 7 days |
30mg/kg |
Every 12 hours |
Max per dose 125mg |
|
Neonate 7-28 days |
30mg/kg |
Every 8 hours |
Max per dose 125mg |
|
Child 1 to 3 months |
30mg/kg |
Every 8 hours |
|
RECONSTITUTION [1,2,4,5]
Intravenous:
- Reconstitute 500mg vial with 4.6mL Water for Injection (displacement volume = 0.4mL) to give a solution containing amoxicillin 100mg/ml.
- Withdraw the required amount and add to a suitable volume of sodium chloride 0.9% or glucose 5% [1].
- Doses up to 150mg (1.5mL) should be diluted to a total volume of 3mL.
- Doses above 150mg (1.5mL) should be diluted with the same volume of a suitable diluent (e.g. 250mg dose – 2.5mL of amoxicillin solution added to 2.5mL of sodium chloride 0.9% or glucose 5%)
- The solution should be colourless to pale straw in colour (a transient pink colour or slight opalescence may appear during reconstitution) and free from particles.
- If glucose 5% is used to further dilute the reconstituted amoxicillin, the solution should be used within one hour as amoxicillin is less stable in infusions containing carbohydrate [2].
Oral: Follow the reconstitution instructions on the pack.
ADMINISTRATION
Intravenous:
- Doses over 30mg/kg must be given by intravenous infusion over 30 - 60 minutes [4].
- If prescribed concurrently with an aminoglycoside, the antibiotics should not be mixed because loss of activity of the aminoglycoside can occur under these conditions. They should preferably be administered at a different site. If this is not possible then the line should be flushed thoroughly with a compatible solution between drugs. [2]
Oral: Give using an appropriate oral/enteral syringe.
SAMPLE CALCULATION
1 day old preterm neonate (0.9kg) with susceptible infection. Amoxicillin 50mg/kg every 12 hours. 50mg x 0.9kg = 45mg every 12 hours. Reconstitute 500mg vial with 4.6mL Water for Injection to give 100mg/mL = 45mg in 0.45mL. Dose below 150mg: dilute to 3mL. Add 0.45mL of the amoxicillin solution to 2.55mL of sodium chloride 0.9%. Infuse at a rate of 0.1mL/min over 30 minutes every 12 hours.
7 day old neonate (3.5kg) with Listerial Meningitis
Amoxicillin: 100mg/kg every 8 hours
100mg x 3.5kg = 350mg every 8 hours
Reconstitute 500mg vial with 4.6mL Water for Injection
500mg in 5mL (displacement value) = 350mg in 3.5mL
Dose above 150mg: dilute with the same volume of a suitable diluent
Add 3.5mL of the amoxicillin solution to 3.5mL of 0.9% Sodium Chloride.
The resulting solution is 7mL
Infuse at a rate of 0.2mL/min over 35 minutes every 8 hours.
STORAGE
Intravenous: Store in original package to protect from moisture/light and keep below 25°C. Use solution immediately following reconstitution [2].
Oral: Dry powder: Do not store above 25°C. Once reconstituted, store in fridge and use within 7 days. [6]
MONITORING
- Renal function should be monitored and doses adjusted in patients with renal impairment [2].
- Maintain adequate hydration and urinary output during high dose administration of amoxicillin in order to reduce the risk of amoxicillin crystalluria [2].
- Monitor for rashes and other skin reactions.
ADVERSE EFFECTS
The main adverse effects seen with amoxicillin administration are diarrhoea, nausea, urticaria, maculopapular rashes, fever, joint pains and angioedema [2].
REFERENCES
- British Medical Association, et al., BNF for Children. Accessed via www.medicinescomplete.com , 16/11/2020 . 2020, BMJ Group and Pharmaceutical Press: London.
- GlaxoSmithKline Ltd., Amoxil Vials 500 mg, powder for solution for injection or infusion, 2018. Available from www.hpra.ie , accessed 16/11/2020.
- Amplifi. Wellness Center: Master Formulary Look Alike Sound Alike Chart . 2008; Available from: http://pharmacyonesource.com/images/amplifi/soundalike.pdf .
- Gray, A., et al., Injectable Drugs Guide- Accessed via www.medicinescomplete.com , 16/11/2020. 2020, Pharmaceutical Press: London.
- Medusa Injectable Drugs Guide. Amoxicillin Intravenous – Paediatric Monograph, 2020. Available from www.medusa.wales.nhs.uk , accessed 16/11/2020.
- Clonmel Healthcare Limited. Summary of Product Characteristics for Amoxicillin 125mg/5mL powder for oral suspension, 2019. Available from www.hpra.ie , accessed 16/11/2020.
Summary of Changes from Previous Versions
|
Date |
Change |
|
Feb 2021: Rev. No. 1 |
Updated based on Rotunda Amoxicillin Monograph Mar 2019. Changes to OLOL monograph:
|
|
Jan 2015 |
This is the first version of this guideline. It is based on the Rotunda Hospital Neonatal Monograph for Amoxicillin, Doc. No. 4, Revision No. 0, date of issue 11/8/14. OLOL changes compared to Rotunda monograph:
|
Neonatal - BenzATHINE benzylpenicillin IM
Neonatal - BenzATHINE benzylpenicillin “1,200,000” IM
Tall Man lettering has been used to differentiate benzaTHINE benzylpenicillin and BENZYLpenicillin.
BenzATHINE benzylpenicillin is a prolonged release penicillin antibiotic used for the treatment of infections related to maternal syphilis serology reactive and confirmed positive [1,2].
MEDICATION SAFETY ISSUES
- A fatal neonatal medication error has been reported after confusion between BENZYLpenicillin and benzATHINE benzylpenicillin [3]. A series of errors led to the IV administration of benzaTHINE benzylpenicillin which should only be administered IM. Inadvertent IV administration has resulted in thrombosis, severe neurovascular damage, cardiac arrest and death [4]. DO NOT GIVE INTRAVENOUSLY.
- It is essential to use the correct needle size when reconstituting and administering this product to reduce the risk of the patient not receiving the full dose [1].
- Avoid concomitant use with BCG vaccine [5].
USES
Used for the treatment of congenital syphilis in infants during the first month of life [2,6].
PRESENTATION
Benzetacil® “1,200,000” powder for solution for Intramuscular injection.
1,200,000 units is equivalent to 900mg of benzATHINE benzylpenicillin.
DOSAGE
Neonatal: Asymptomatic congenital syphilis: Intramuscular 50,000 units/kg as a single dose [2,5].
BenzATHINE benzylpenicillin 50,000 units/kg = 37.5 mg/kg [2].
RECONSTITUTION
The reconstituted suspension must be used immediately.
Using a needle size of 20G (available in NICU), reconstitute the Benzetacil® 1,200,000 unit vial with 3mL of the solvent provided to give 1,200,000 units in 3mL (400,000 units per mL).
ADMINISTRATION
The injection should be reconstituted immediately before use. The needle may become blocked if injection is not made at a slow, steady rate due to high concentration of suspended material in the product [6].
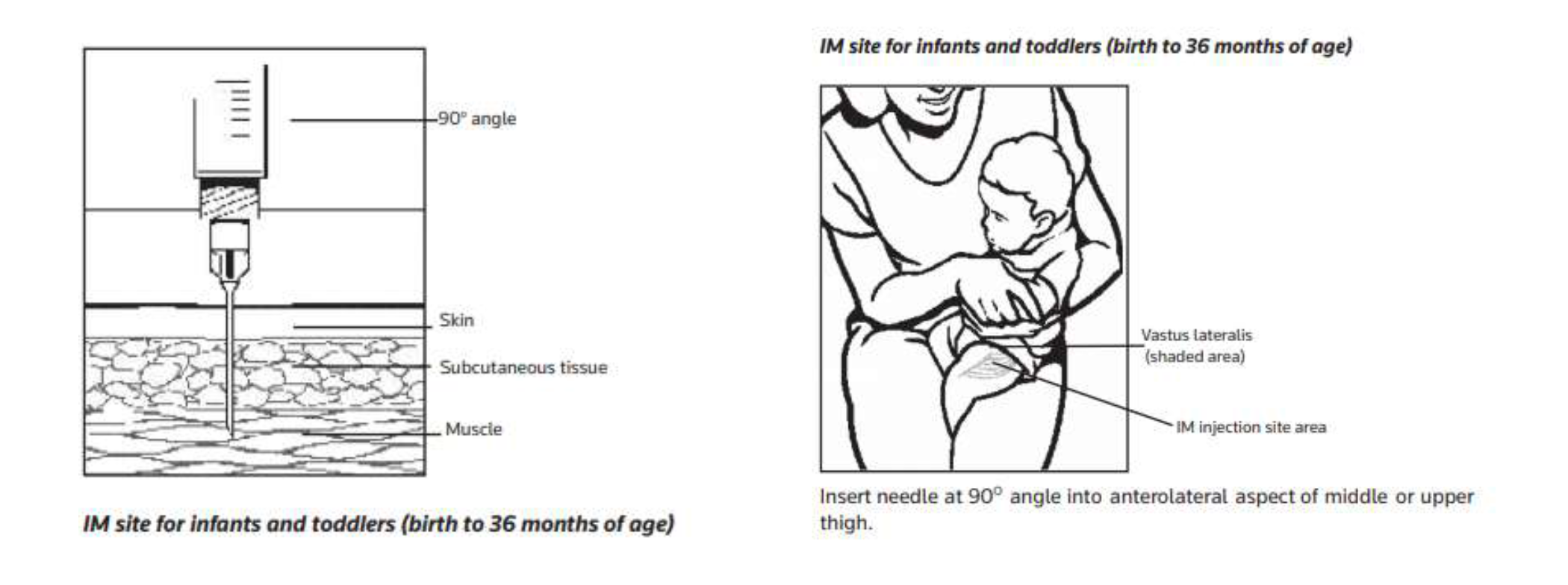
Administer the correct dose using the reconstituted solution immediately via the intramuscular route using a 20G needle . Using a smaller diameter needle may lead to blockages and failed injection attempts. Avoid injection into or near an artery or nerve.
Paracetamol for pain relief should be given prior to administration. Oral sucrose should be given for comfort throughout treatment as required.
SAMPLE CALCULATION
Case: 3.28 kg baby with asymptomatic congenital syphilis.
Dose: 50,000 units per kg = 50,000 x 3.28 = 164,000 units.
When reconstituted, benzATHINE benzylpenicillin contains 1,200,000 units in 3mL.
Dose =
What you want X Volume it is in
What you have got
= 164,000 units X 3mL
1,200,000 units
= 0.41mL
STORAGE
Use immediately once reconstituted.
MONITORING
BenzATHINE benzylpenicillin is renally excreted. In young infants and patients with renal impairment, excretion is prolonged [6].
ADVERSE EFFECTS
Hypersensitivity reactions may occur [1,4].
REFERENCES
- Benzetacil Patient Information Leaflet S.A. Laboratorio Reig Jofre, Editor.
- The Rainbow Clinic 2015, Preventing Perinatal Transmission: A Practical Guide to the antenatal and perinatal management of HIV, Hepatitis B, Hepatitis C, Herpes Simplex & Syphilis, Our Lady’s Children’s Hospital, Crumlin and The Children’s University Hospital, Temple Street , Dublin.
- Institute for Safe Medication Practices, A case riddled with latent and active failures. ISMP Medication Safety Alert!, 1998(February 11): p. URL:http://www.ismp.org/newsletters/acutecare/articles/19980211_2.asp?ptr=y. Accessed: 23rd April 2014.
- Health, W.K. UpToDate clinical decision support resource, Penicillin G Benzathine (Intramuscular) Pediatric drug information. 2020; Available from: http://www.uptodate.com . Accessed 17/11/2020.
- Takemoto Carol, e.a., Pediatric and Neonatal dosage handbook 18 ed, ed. Lexicomp. 2011-2012.
- Reuters, T., Neofax A manual of drugs used in neonatal care. Vol. 24. 2011.
Summary of Changes from Previous Versions
|
Date |
Change |
|
Feb 2021: Rev. No. 1 |
Updated based on Rotunda BenzATHINE benzylpenicillin Monograph Nov 2018. Changes to OLOL monograph:
|
|
Apr 2015 |
This is the first version of this guideline. It is based on the Rotunda Hospital Neonatal Monograph for Benzathine penicillin, Doc. No. 1, Revision No. 0, date of issue 10/11/14. Changes in OLOL monograph compared to the Rotunda monograph:
|
Neonatal - Benzylpenicillin IV
Neonatal - BENZYLpenicillin sodium IV (Penicillin G)
Bactericidal beta-lactamase sensitive penicillin
MEDICATION SAFETY ISSUES
- A fatal neonatal medication error has been reported after confusion between BENZYLpenicillin and benzATHINE benzylpenicillin [1]. A series of errors led to the IV administration of benzATHINE benzylpenicillin which should only be administered IM.
- Anaphylaxis has been rarely reported in neonates after exposure to beta-lactam antibiotics. [2]
USES
Empiric treatment of suspected neonatal sepsis. Treatment of mild to moderately severe infections such as pneumonia and cellulitis, as well as more severe infections such as endocarditis and meningitis due to penicillin susceptible organisms.
PRESENTATION
BENZYLpenicillin is available as Crystapen®. Each vial contains 600mg BENZYLpenicillin sodium.
Doses are expressed in terms of the sodium salt. Each vial contains 1.68 mmol sodium.
DOSAGE [3,4,7]
|
Dose and frequency of BENZYLpenicillin |
Neonatal sepsis (LP not done) |
Group B Strep Meningitis [3] |
Endocarditis (seek advice, combination therapy may be needed) [4] |
|
Dose |
|||
|
All ages |
50mg/kg |
75mg/kg |
50mg/kg |
|
Frequency |
|
||
|
Neonate under 7 days |
Every 12 hours |
- |
|
|
Neonate 7-28 days |
Every 8 hours |
- |
|
|
Child > 28 days |
Every 4 - 6 hours |
Every 4 hours |
|
Renal Impairment [4 ]
Dose reduction may be required in renal impairment refer to BNFc and seek advice on assessment of renal function.
RECONSTITUTION [5,7]
Reconstitute the 600mg amp with 5.6mL of water for injection (displacement volume = 0.4mL) to give a solution containing 100mg/mL [5]. May be diluted with sodium chloride 0.9% or glucose 5% if an infusion is required.
ADMINISTRATION
Give by IV infusion over 15 - 30 minutes. Longer administration time is particularly important when using doses of 50mg/kg or greater to avoid CNS toxicity [4].
SAMPLE CALCULATION
2.8kg neonate under 7 days with suspected meningitis. Dose: 75 mg/kg every 12 hours = 210mg every 12 hours. Reconstitute 600mg vial with 5.6mL water for injections = 100mg/mL.
Withdraw 2.1mL of the resultant solution and dilute to an appropriate volume for that baby (e.g. 10mL) with sodium chloride 0.9% or glucose 5% and administer over 15-30 minutes.
STORAGE
Store at room temperature below 25 o C. Degradation or transformation of BENZYLpenicillin occurs rapidly and may be responsible for sensitisation. Reconstituted solutions should be used immediately [6].
MONITORING
Electrolyte balance, blood counts and renal functions should be monitored if treatment is prolonged (more than 5 days) [7]. Hypernatraemia and hypokalaemia may occur with high doses.
ADVERSE EFFECTS [4 ]
Hypersensitivity reactions including urticaria, fever, joint pains, rashes, angioedema, anaphylaxis, serum sickness-like reactions; rarely CNS toxicity including convulsions (especially with high doses or in severe renal impairment), interstitial nephritis, haemolytic anaemia, leucopenia, thrombocytopenia and coagulation disorders; also reported diarrhoea (including antibiotic-associated colitis).
REFERENCES
1. Institute for Safe Medication Practices, A case riddled with latent and active failures. ISMP Medication Safety Alert!, 1998(February 11): p. URL: http://www.ismp.org/newsletters/acutecare/articles/19980211_2.asp?ptr=y . Accessed: 23rd April 2014.
4. British Medical Association, et al., BNF for Children. Accessed via www.medicinescomplete.com , 17/11/2020 . 2020, BMJ Group and Pharmaceutical Press: London.
6. Phelps, S.J., E.B. Hak, and C.M. Crill, Pediatric Injectable Drugs. 9th ed. Accessed via www.medicinescomplete.com March 31st 2014 . 2010, American Society of Health-System Pharmacists: Bethesda.
Summary of Changes from Previous Versions
|
Date |
Change |
|
Feb 2021: Rev. No. 1 |
Updated based on Rotunda BENZYLpenicillin Sodium Monograph Feb 2019. Changes to OLOL monograph:
|
|
Nov 2014 |
This is the first version of this guideline. It is based on the Rotunda Hospital Neonatal Monograph for Benzylpenicillin, Doc. No. 2, Revision No. 0, date of issue 19/5/14. There is one change in the OLOL monograph compared to the Rotunda monograph – Administration of 50mg/kg dose of benzylpenicillin as an IV infusion as per BNF for Children 2014. Additional references have also been added to the dosing section (no change to dose). |
Neonatal - Cef-O-taxime IV
Neonatal - Cef-O-taxime IV
Broad-spectrum third generation bactericidal cephalosporin antibiotic.
MEDICATION SAFETY ISSUES
Confusion with other cephalosporins e.g. Cef-TRI-axone and Cef-UR-oxime.
Should not be used in patients with a known allergy to a cephalosporin.
Individuals who have a hypersensitivity reaction to penicillin may have cross sensitivity to cephalosporins.
USES
Meningitis and severe neonatal infections.
PRESENTATION
Cef-O-taxime 1 g Wockhardt, Claforan® 1g Sanofi.
DOSAGE [1]
Neonates: Meningitis and Severe Infections
|
Age |
Dose |
Frequency |
|
Neonate under 7 days |
50mg/kg |
Every 12 hours |
|
Neonate 7-21 days |
50mg/kg |
Every 8 hours |
|
>21 days |
50mg/kg |
Every 6 hours |
For less severe infection doses of 25 mg/kg/dose have been used for neonates.
Renal Impairment [1 ]
Dose reduction may be required in renal impairment refer to BNFc and seek advice on assessment of renal function.
RECONSTITUTION [4,7]
Cef-O-taxime is available as a dry powder containing 1 gram per vial.
To prepare Cef-O-taxime solution 100 mg/mL:
To each vial of Cef-O-taxime add appropriate volume of Water for Injection as per table below and shake well to mix to give Cef-O-taxime 100 mg/mL solution.
This can be further diluted with sodium chloride 0.9% or glucose 5% if required.
|
Brand |
Approx displacement volume |
Diluent to be added |
Final Concentration |
|
Cef-O-taxime 1g Wockhardt |
0.6mL |
9.4mL |
100mg/mL |
|
Cef-O-taxime 1g Claforan® |
0.4mL |
9.6mL |
100mg/mL |
ADMINISTRATION
Give by slow IV injection over 3 - 5 minutes or by IV infusion over 20 - 60 minutes [1].
(Rapid IV push < 1 minute has been associated with life threatening arrhythmias) [1].
Should not be mixed in the same syringe with an aminoglycoside – flush IV line well between the administration of cef-O-taxime and an aminoglycoside to avoid formation of precipitate.
SAMPLE CALCULATION
1.3kg neonate 8 days old with suspected meningitis.
Dose: 50mg/kg every 8 hours = 65mg every 8 hours.
Reconstitute 1g vial (Wockhardt) with 9.4mL WFI as per table above to make 100mg/mL solution
Withdraw 0.65mL of the resultant solution and dilute to an appropriate volume for that baby (e.g. 5mL) with sodium chloride 0.9% or glucose 5% and administer over 20-60 minutes.
STORAGE
Store vials in original outer carton at room temperature. Discard remaining reconstituted solution after dose has been given.
MONITORING
Monitor serum creatinine and urea in neonates who are also receiving aminoglycosides and furosemide.
Dosage interval should be increased in babies with severe renal failure (serum creatinine >200 mmol/L).
Monitor for diarrhoea in infants on prolonged therapy.
SIDE EFFECTS
Candidiasis, rash, fever, transient rises in liver transaminase and/or alkaline phosphatase and diarrhoea. Pseudomembranous colitis may rarely occur. Eosinophilia, neutropenia,throbocyropenia and haemolytic anaemia have been reported also – if treatment for longer than 10 days, monitor full blood count. Transient pain may be experienced at the injection site- administration as an infusion will reduce pain.
REFERENCES
- British National Formulary for Children (BNFc). Available from www.medicinescomplete.com , accessed 17/11/2020.
- Neonatal Formulary 6. The Northern Neonatal Network Sixth Edition 2011.
- Children’s Health Ireland. Paediatric Formulary. Cefotaxime Monograph. Available via smartphone application, accessed 17/11/2020.
- Summary of Product Characteristics Cefotaxime for Injecton or Infusion Wockhardt. Last updated Sep 2017. Available from www.hpra.ie , accessed 17/11/2020.
- Lexicomp Paediatric 7 Neonatal Dosage Handbook 18 th Edition 2011.
- Pediatric Injectable Drugs – The Teddy Bear Book Ninth Edition.
- Letter communication from Sanofi Aventis received February 2014: displacement value for Claforan®.
Summary of Changes from Previous Versions
|
Date |
Change |
|
Feb 2021: Rev. No. 2 |
Updated based on Rotunda Cef-O-taxime Monograph Feb 2019. Changes to OLOL monograph:
|
|
Mar 2018: Rev. No. 1 |
Displacement value for cef-O-taxime 1g (Wockhardt) updated as per SPC update. Claforan® strength and displacement value updated as 500mg vials no longer stocked in OLOL, 1g vials stocked if needed. |
|
Nov 2014 |
This is the first version of this guideline. It is based on the Rotunda Hospital Neonatal Monograph for Cefotaxime, Doc. No. 3, Revision No. 0, date of issue 19/5/14. There are four changes in the OLOL monograph compared to the Rotunda monograph:
|
Neonatal - Flucloxacillin IV
Neonatal - Flucloxacillin IV
Flucloxacillin is a bactericidal penicillin antibiotic which is effective against staphylococci. Flucloxacillin is acid stable and can be given by mouth where appropriate. [1]
MEDICATION SAFETY ISSUES
- Special caution is essential in the newborn and neonates with renal impairment because of the potential for high serum levels of flucloxacillin due to a reduced rate of renal excretion.[2]
- Risk of kernicterus in jaundiced neonates when high doses given parenterally. [2]
- Cholestatic jaundice and hepatitis may occur very rarely, up to two months after treatment with flucloxacillin has stopped. Administration for more than 2 weeks and increasing age are risk factors. [1]
USES
Infections caused by beta-lactamase-producing staphylococci including otitis externa; as an adjunct in pneumonia, cellulitis and osteomyelitis and in staphylococcal meningitis and endocarditis [1].
PRESENTATION
Powder for solution for injection or infusion: Floxapen® 250mg and 500mg vials [2]
DOSAGE [1,3]
|
Age |
Dose |
Frequency |
Max Dose |
|
Neonate <7 days |
50mg/kg |
Every 12 hours |
|
|
Neonate 7 – 21 days |
50mg/kg |
Every 8 hours |
|
|
Neonate 21 – 28 days |
50mg/kg |
Every 6 hours |
|
|
Child 1 to 3 months |
25mg/kg |
Every 6 hours |
Max 1g every 6 hours |
Dose may be doubled for severe infections including osteomyelitis, endocarditis or suspected/confirmed staphylococcal meningitis [3]
RECONSTITUTION [3-6]
- 250mg vial : Reconstitute 250mg vial with 4.8mL WFI (Displacement volume 0.2mL) to give a final concentration of 50mg/mL.
- 500mg vial : Reconstitute 500mg vial with 4.7mL WFI (Displacement volume 0.3mL) to give 100mg/mL. Further dilute 5mL of this solution with 5mL of Sodium Chloride 0.9% or Glucose 5% (to a final volume of 10mL). The resulting solution contains flucloxacillin 50mg/mL.
ADMINISTRATION
Administer as either a slow IV injection over 3 – 5 minutes OR Add the required dose to a suitable volume of diluent (e.g. sodium chloride 0.9% or glucose 5%) and give as an IV infusion over 30 minutes [1,6].
SAMPLE CALCULATION
Two week old preterm neonate (1.1kg) with staphylococcal meningitis.
Dose = 100mg/kg every 8 hours = 110mg every 8 hours.
Reconstitute 250mg vial with 4.8ml WFI to give a concentration of 50mg/mL.
Withdraw 2.2mL (110mg) and give either as a slow IV injection or IV infusion over 30 minutes.
STORAGE
Do not store above 25 o C. Once reconstituted, the product should be used immediately. [2]
MONITORING [1]
- Monitor liver function and renal function if high doses or prolonged courses.
- Hepatic disorders: Cholestatic jaundice may occur up to 2 months after treatment has stopped. Risk is increased with prolonged courses and increasing age.
- Renal impairment: Dosage reduction not usually required, use normal dose every 8 hours if severe renal impairment.
ADVERSE EFFECTS
Hypersensitivity reactions including urticaria, fever, joint pains, rashes, angioedema, anaphylaxis, serum sickness-like reactions; rarely CNS toxicity including convulsions (especially with high doses or in severe renal impairment), interstitial nephritis, haemolytic anaemia, leucopenia, thrombocytopenia and coagulation disorders; also reported diarrhoea (including antibiotic-associated colitis); also gastro-intestinal disturbances; very rarely hepatitis and cholestatic jaundice reported. [1]
REFERENCES
- British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health, et al. BNF for Children. London: BMJ Group, Pharmaceutical Press and RCPCH Publications Limited; 2020. Available from www.medicinescomplete.com , accessed 17/11/2020.
- Actavis Group. Floxapen 250mg Powder for solution for Injection or Infusion. 2018. Available from www.hpra.ie , accessed 17/11/2020.
- Children’s Health Ireland. Paediatric Formulary. Flucloxacillin Monograph. Available via smartphone application, accessed 17/11/2020.
- Department of Health and Government of South Australia. South Australia Neonatal Medication Guidelines – Flucloxacillin 2018. Available from http://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/clinical+resources/clinical+topics/neonatal+medication+guidelines , accessed 17/11/2020.
- Our Lady of Lourdes Hospital. Displacement values for injectable medicines, Oct 2019. On file in Pharmacy Department Z Drive, OLOL.
- Medusa Injectable Drugs Guide. Flucloxacillin Intravenous – Paediatric Monograph, 2020. Available from www.medusa.wales.nhs.uk , accessed 17/11/2020.
Summary of Changes from Previous Versions
|
Date |
Change |
|
Feb 2021: Rev. No. 1 |
Updated based on Rotunda Flucloxacillin Monograph Mar 2019. Changes to OLOL monograph:
|
|
Jan 2015 |
This is the first version of this guideline. It is based on the Rotunda Hospital Neonatal Monograph for Flucloxacillin, Doc. No. 1, Revision No. 0, date of issue 10/11/14. Changes in OLOL monograph compared to the Rotunda monograph:
|
Neonatal - Fluconazole IV and PO
Neonatal - Fluconazole IV and PO
Triazole antifungal [1].
MEDICATION SAFETY ISSUES
- Fluconazole may be confused with other azole antifungals [2]
USES
- Treatment of susceptible invasive candidal infections and cryptococcal infections [1].
- Prophylaxis of fungal infections in infants <1kg with risk factors for invasive candida such as broad spectrum antimicrobials and NEC. Prophylaxis considered on a case by case basis in patients >1kg with risk factors. Under Consultant direction [3,4].
PRESENTATION
Intravenous : Solution for infusion 2mg/ml [5]. Each 200mg bottle contains 15mmol of sodium [1,5].
Oral: Powder for oral suspension.
TREATMENT DOSAGE [1,5]
|
Age |
Dose |
Frequency |
Duration |
|
Neonate under 14 days |
6 – 12 mg/kg |
Every 72 hours |
Continue according to response (at least 8 weeks for cryptococcal infections) |
|
Neonate 14 – 28 days |
6 – 12 mg/kg |
Every 48 hours |
|
|
Child 1 – 3 months |
6 – 12 mg/kg |
Every 24 hours |
PROPHYLACTIC DOSAGE: 3mg/kg/dose every 72 hours for 6 weeks by oral or intravenous administration [3,4]
Renal Impairment : Dose reduction may be required – refer to BNFc and seek advice [1].
RECONSTITUTION
Intravenous: Fluconazole solution for infusion is already in liquid form, further dilution is not necessary [1,5].
Oral: For reconstitution of the oral suspension follow the instructions on the pack.
ADMINISTRATION
Fluconazole may be administered either orally or by intravenous infusion, the route being dependent on the clinical state of the patient. On transferring from the intravenous to the oral route, or vice versa , there is no need to change the dose [1].
Oral: Shake the bottle before use. Administer using an oral/enteral syringe.
Intravenous: Administer by IV infusion over 30 minutes; do not exceed an infusion rate of 5 – 10 ml/min.[1]
SAMPLE CALCULATION FOR TREATMENT
3.2 kg neonate 9 days old with susceptible candida infection. Dose: 12mg/kg = 38.4mg every 72 hours.
Fluconazole 2mg/ml solution = 38.4mg in 19.2ml. Withdraw 19.2ml and give by IV infusion over 30 min.
SAMPLE CALCULATION FOR PROPHYLAXIS
5 day old, 0.8 kg extremely preterm infant born at 26 weeks gestational age on parenteral nutrition and broad-spectrum antibiotics. Dose (as advised by Consultant): 3 mg/kg = 2.4 mg every 72 hours.
Fluconazole 2mg/ml solution = 2.4 mg in 1.2 ml. Withdraw 1.2 ml and give by IV infusion over 30 min.
STORAGE
Intravenous: Once opened, use immediately. Any unused infusion should be discarded.[5]
Oral: The shelf life of the reconstituted suspension is 28 days. Store below 30°C, do not freeze [6].
MONITORING [4]
- Fluconazole may interact with Erythromycin, Ibuprofen, Midazolam , Phenytoin, Zidovudine. This list is not complete. Always check for drug interactions.
- Monitor patient for rash – discontinue fluconazole if bullous lesions or erythema multiforme develop.
- Monitor renal function – dose reduction may be required in renal impairment.
- Monitor liver function – risk of hepatic toxicity.
ADVERSE EFFECTS
The most frequently (>1/10) reported adverse reactions are headache, abdominal pain, diarrhoea, nausea, vomiting, ALT increased, AST increased, ALP increased and rash [5]. Less frequently dyspepsia, taste disturbance, hepatic disorders, angioedema, anaphylaxis, dizziness, seizures, alopecia, pruritus, toxic epidermal necrolysis, Stevens-Johnson syndrome, hyperlipidaemia, leucopenia, thrombocytopenia, and hypokalaemia [1].
REFERENCES
- British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health, et al. BNF for Children. London: BMJ Group, Pharmaceutical Press and RCPCH Publications Limited; 2020. Available from www.medicinescomplete.com , accessed 23/11/2020.
- UpToDate. Fluconazole Paediatric Drug Information: Lexicomp®. Available from www.uptodate.com , accessed 08/12/14.
- Hope, W.W., et al., ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect, 2012. 18 Suppl 7 : p. 38-52.
- Pappas, P.G., et al., Executive Summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases, 2016. 62 (4): p. 409-417.
- B.Braun. Summary of Product Characteristics for Fluconazole 2mg/mL Solution for Infusion. 2017. Available from www.hpra.ie , accessed 23/11/2020.
- Pfizer. Patient Information Leaflet for Diflucan® powder for oral suspension. 2020. Available from www.hpra.ie , accessed 23/11/2020.
Summary of Changes from Previous Versions
|
Date |
Change |
|
Feb 2021: Rev. No. 1 |
Updated based on Rotunda Fluconazole Monograph May 2018. Changes to OLOL monograph:
|
|
May 2015 |
This is the first version of this guideline. |
Neonatal - Gentamicin IV
Neonatal - Gentamicin IV – Fresenius Kabi USA Brand, Mar 2025
Aminoglycoside antibiotic of choice [1].
MEDICATION SAFETY ISSUES
- Extended-interval regimen used for neonates – check dose interval carefully , monitor trough levels .
- The National Patient Safety Agency (NPSA) issued a patient safety alert in 2010 on the safer use of intravenous gentamicin in neonates [2]. A review of neonatal medication incidents identified that gentamicin was involved in 15% of all reported neonatal medication incidents. The most frequently reported incident (36%) related to administration at the incorrect time.
- Following reports that some batches of gentamicin sulphate active pharmaceutical ingredient (API) used to manufacture gentamicin may contain higher than expected levels of histamine, which is a residual from the manufacturing process, monitor patients for signs of histamine-related adverse reactions; particular caution is required in patients taking concomitant drugs known to cause histamine release, in children, and in patients with severe renal impairment [1].
- Contraindicated in mothers and newborn babies of mothers with Myasthenia Gravis . Aminoglycosides may impair neuromuscular transmission.
- Caution in babies undergoing therapeutic cooling due to reduced renal function. Seek Consultant advice before prescribing.
USES
Neonatal sepsis and other severe infections when indicated [1].
PRESENTATION
Gentamicin Pediatric Fresenius Kabi USA, 20mg/2ml ampoules [3].
DOSAGE [1]
|
Age |
Dose |
Frequency |
|
Neonate less than 7 days after birth |
5mg/kg |
Every 36 hours |
|
Neonate more than 7 days after birth |
5mg/kg |
Every 24 hours |
Caution - the main adverse effects of gentamicin (nephrotoxicity and ototoxicity) are dose related. Whenever possible, treatment should not exceed 5 days to minimise risk of toxicity [1].
Renal Impairment : Refer to BNFc and seek advice – change in dosing interval may be required [1].
RECONSTITUTION
- The 20mg/2mL vials are already in liquid form.
- The Fresenius Kabi USA brand must be diluted before use [3].
- Further dilute 20mg with 8mL of sodium chloride 0.9% or glucose 5% to a final volume of 10mL [3].
- The resulting solution contains gentamicin 2mg/mL.
- In the case of fluid restriction, a more concentrated dilution may be appropriate, contact Consultant Neonatologist for advice. The product licence for Fresenius Kabi Gentamicin 20mg/2mL does not specify the level of dilution required.
ADMINISTRATION
Administer by IV infusion over 30 minutes [3].
SAMPLE CALCULATION
3.5kg neonate < 7 days after birth with queried sepsis. Dose 5mg/kg every 36 hours = 17.5mg.
Vial contains 20mg/2mL. Further dilute 20mg with 8mL sodium chloride 0.9% or glucose 5% to a final volume of 10mL. The resulting solution contains 2mg/mL.
Withdraw 8.75mL (17.5mg) and give over 30 minutes.
STORAGE
Store unopened vials at room temperature 20 o C to 25 o C [3]. Discard any unused portions [3].
MONITORING
- Risk of nephrotoxicity - maintain adequate hydration and monitor renal function closely [1]. Caution if concomitant administration of other nephrotoxic medicines.
- Risk of ototoxicity - review concomitant ototoxic medicines [1]. If furosemide required (potentially ototoxic), separate administration from gentamicin by as long a period as practicable.
- Monitor gentamicin trough levels as directed below.
TROUGH LEVEL MONITORING
- Check trough level immediately before next dose is due.
- Document the date/time of blood sample and the date/time of last dose administered clearly on the laboratory request form. This will allow accurate interpretation of trough level results.
When to check first trough level [4-6]
|
Neonate Category |
First Trough Level |
Comment |
|
Normal renal function / No concern regarding renal function (for example, urea and creatinine normal, urine output > 1ml/kg/hour) |
Before 3 rd dose |
Give 3 rd dose without waiting for trough level result. |
|
Reduced urine output or uncertainty of adequate renal clearance Examples:
|
Before 2 nd dose |
Discuss with Consultant Neonatologist whether to give 2 nd dose or wait for trough level result before giving dose. Risk/benefit decision – treatment of acutely septic neonate versus concern over renal function. |
|
Corrected gestational age < 32 weeks and more than 7 days after birth , prescribed gentamicin every 24 hours |
Before 2 nd dose |
Give 2 nd dose without waiting for trough level result. Previously, this patient group received gentamicin every 36 hours, therefore with shortened dosing interval of 24 hours, it is prudent to check trough level pre-2 nd dose. |
Interpretation of trough level result [1,6]
|
Trough |
Action |
|
Acceptable: < 2mg/L (< 1mg/L if more than 3 doses given) |
Continue same gentamicin dose and dose-interval. |
|
High: > 2mg/L ( > 1mg/L if more than 3 doses given) |
Hold next dose. Repeat trough level 12 hours later. If level is due out-of-hours, please contact biochemistry laboratory staff in advance to arrange processing of the result. Wait for trough level result before giving a further dose:
|
When to check subsequent trough levels
|
Neonate Category |
Subsequent Trough Levels |
|
Reduced urine output or uncertainty of adequate renal clearance |
Before every dose or every alternate dose (Consultant decision) |
|
Normal renal function |
Before every third dose |
ADVERSE EFFECTS
The important adverse effects of the aminoglycosides are nephrotoxicity and irreversible ototoxicity (including vestibular and auditory damage) [1]. See BNF for Children [1] or Summary of Product Characteristics [3] for further information on possible adverse effects.
REFERENCES
- British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health, et al. BNF for Children. London: BMJ Group, Pharmaceutical Press and RCPCH Publications Limited; 2020. Available from www.medicinescomplete.com , accessed 23/11/2020.
- National Patient Safety Agency (NPSA). Safer use of intravenous gentamicin for neonates. Patient Safety Alert! NPSA/2010/PSA001. Available from www.npsa.nhs.uk , accessed 10/12/14.
- Fresenius Kabi USA, LLC. Summary of Product Characteristics for Gentamicin Pediatric 20mg/2ml Solution for Injection. 2023. On file in OLOL Pharmacy, Mar 2025.
- Department of Health and Government of South Australia, South Australian Neonatal Medication Guidelines - Gentamicin 2017. Available from http://www.sahealth.sa.gov.au/wps/wcm/connect/34c75d004cd7d772b93bb9a496684d9f/gentamicin_Mar2015.pdf?MOD=AJPERES&CACHEID=34c75d004cd7d772b93bb9a496684d9f , accessed 23/11/2020.
- Ainsworth SB. Neonatal Formulary 7: Web Commentary for Gentamicin. Wiley Blackwell & BMJ Books. Updated Jul 2014. Available from http://www.neonatalformulary.com , accessed 26/11/18.
- Gentamicin Project Improvement Group as part of the National Quality Improvement Programme, HSE/RCPI. Gentamicin Guidelines for once daily usage in adult and paediatric settings. 2016. Available from www.rcpi.ie .
Summary of Changes from Previous Versions
|
Date |
Change |
|
Mar 2025: Rev. No. 2 |
Due to stock shortage, switch to Fresenius Kabi USA brand of gentamicin pediatric 20mg/2mL (unlicensed). SPC specifies it must be diluted before IV use. Dilution to 2mg/mL as per practice in Coombe and Holles St. Hospitals. |
|
Feb 2021: Rev. No. 1 |
Updated based on Rotunda Gentamicin Monograph Feb 2019. Changes to OLOL monograph:
|
|
August 2015 |
This is the first version of this guideline. |
Neonatal - Meropenem IV
Neonatal - Meropenem IV
Meropenem is a broad-spectrum carbapenem antibiotic [1]. It is a restricted antimicrobial in LH and should only be prescribed following consultation with the Clinical Microbiologist.
MEDICATION SAFETY ISSUES
- Meropenem is a beta-lactam antibiotic. Avoid if history of immediate hypersensitivity reaction to beta-lactam antibacterials (e.g. penicillins or cephalosporins). Use with caution in patients with sensitivity to beta-lactam antibacterials [2,3].
- Incompatible with Aciclovir, Amphotericin B, Calcium Gluconate, Metronidazole, Sodium Bicarbonate and Zidovudine [ 1 ].
USES
Treatment of multi-drug resistant infection caused by certain gram-negative and gram-positive organisms, hospital-acquired septicaemia and meningitis [2]. Not licensed for use in children < 3 months of age [3].
PRESENTATION
Meropenem 500 mg Powder for Solution for Injection or Infusion [6].
DOSAGE [3,4]
|
Age |
Dose |
Interval |
|
Neonate <7 days |
40mg/kg |
Every 12 hours |
|
Neonate 7 – 28 days |
40mg/kg |
Every 8 hours |
|
Child 1 to 3 months |
40mg/kg |
Every 8 hours |
RECONSTITUTION [3,7]
NB. There are TWO STEPS for reconstitution. BOTH Step 1 and Step 2 below must be followed.
Step 1 : Reconstitute 500mg vial with 9.5mL WFI (Displacement volume 0.5mL) to give 50mg/mL. The displacement value of 0.5mL is for Hikma/Demo/Noridem brands (Fannin) of Meropenem 500mg. Check the displacement value if a different brand is used.
Step 2 : Further dilute 4mL of this solution with 6mL sodium chloride 0.9% or glucose 5% to a final volume of 10mL. The resulting solution contains meropenem 20mg/mL.
ADMINISTRATION
Administer as an IV infusion over 30 minutes.
SAMPLE CALCULATION
A 10 day old baby with meningitis weighing 2.3kg. Dose = 40mg/kg = 92mg every 8 hours.
Reconstitue meropenem as above to a solution containing 20mg/mL.
Withdraw 4.6mL (92mg) and administer by IV infusion over 30 minutes.
STORAGE
After reconstitution the solution should be used immediately. The time interval between the beginning of reconstitution and the end of intravenous infusion should not exceed 1 hour [6].
MONITORING
- Monitor renal, hepatic, and hematologic function tests [8].
- Dose reduction may be required in renal impairment – refer to BNFc and seek advice [3].
- Observe for changes in bowel frequency (Pseudomembranous colitis has been reported with the use of meropenem) [ 4 ].
ADVERSE EFFECTS
Nausea, vomiting, diarrhoea (antibiotic-associated colitis reported), abdominal pain, disturbances in liver function tests, headache, thrombocythaemia, rash, pruritus; less commonly paraesthesia, eosinophilia, thrombocytopenia, leucopenia; rarely convulsions; also reported haemolytic anaemia, positive Coombs’ test, Stevens-Johnson syndrome, toxic epidermal necrolysis [3].
REFERENCES
- Reuters T. Neofax: A manual of drugs used in neonatal care. Vol. 24, 2011.
- Department of Health and Government of South Australia. South Australia Neonatal Medication Guidelines – Meropenem 2018. Available from http://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/clinical+resources/clinical+topics/neonatal+medication+guidelines , accessed 23/11/2020.
- British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health, et al. BNF for Children. London: BMJ Group, Pharmaceutical Press and RCPCH Publications Limited; 2020. Available from www.medicinescomplete.com , accessed 23/11/2020.
- Children’s Health Ireland. Paediatric Formulary. Meropenem Monograph. Available via smartphone application, accessed 23/11/2020.
- Takemoto C. Paediatric and neonatal dosage handbook. 18 th Edition. Lexicomp, 2011 – 2012.
- Noridem Enterprises Limited (Fannin). Summary of Product Characteristics for Meropenem 500mg powder for solution for injection or infusion. 2018. Available from www.hpra.ie , accessed 23/11/2020.
- Medusa Injectable Drugs Guide. Meropenem Intravenous – Paediatric Monograph, 2018. Available from www.medusa.wales.nhs.uk , accessed 23/11/2020.
- Gray A et al. Injectable Drugs Guide. London: Pharmaceutical Press; 2020. Available from www.medicinescomplete.com , accessed 23/11/2020.
Summary of Changes from Previous Versions
|
Date |
Change |
|
Feb 2021: Rev. No. 2 |
Updated based on Rotunda Meropenem Monograph Mar 2019. Changes to OLOL monograph:
|
|
June 2017: Rev. No. 1 |
Meropenem Hikma Fannin brand stocked in OLOL. Displacement value changed to 0.5ml and calculation updated. |
|
Jan 2015 |
This is the first version of this guideline. It is based on the Rotunda Hospital Neonatal Monograph for Meropenem, Doc. No. 1, Revision No. 0, date of issue 10/11/14. Changes in OLOL monograph compared to the Rotunda monograph:
|
Neonatal - Metronidazole IV
Neonatal - MetroNIDAZOLE IV
Antimicrobial with activity against anaerobic bacteria and protozoa [1].
MEDICATION SAFETY ISSUES
- Anaphylaxis has been reported rarely with metronidazole [2].
- Sound-alike/Look-alike issues: Metronidazole may be confused with metformin, meropenem [3,4].
USES
Infections for which anaerobic cover required, such as Necrotising Enterocolitis (NEC) [1].
PRESENTATION
Metronidazole 5mg/ml Solution for Infusion [2]
DOSAGE [1]
|
Age |
Dose |
Frequency |
|
Neonate less than 26 weeks corrected gestational age |
Single loading dose 15mg/kg followed after 24 hours by 7.5mg/kg each dose |
Every 24 hours |
|
Neonate 26 - 34 weeks corrected gestational age |
Single loading dose 15mg/kg followed after 12 hours by 7.5mg/kg each dose |
Every 12 hours |
|
Neonate over 34 weeks corrected gestational age |
Single loading dose 15mg/kg followed after 8 hours by 7.5mg/kg each dose |
Every 8 hours |
|
Child 1 - 2 months
|
Single loading dose 15mg/kg followed after 8 hours by 7.5mg/kg each dose |
Every 8 hours |
|
Child 2 – 3 months |
7.5mg/kg (max 500mg) |
Every 8 hours |
Hepatic Impairment
Dose reduction may be required in hepatic impairment [1]. Refer to BNFc and seek advice on assessment of liver function [1].
RECONSTITUTION
Metronidazole 5mg/ml solution for infusion is already in liquid form. There is no need to further dilute the infusion solution [2,5,6].
ADMINISTRATION
Give by IV infusion over one hour [1,2,5,7].
SAMPLE CALCULATION
2.8kg neonate corrected gestational age 40 weeks with suspected NEC.
Dose 15mg/kg as a single loading dose followed after 8 hours by 7.5mg/kg every 8 hours.
- 15mg/kg = 42mg. Withdraw 8.4ml metronidazole from the bag and give over one hour.
- 7.5mg/kg = 21mg. Withdraw 4.2ml metronidazole from the bag and give over one hour.
STORAGE
Store at room temperature below 25 o C. Keep container in the outer carton to protect from light. Once infusion solution opened, it must be used immediately. Discard any unused portions. [2,5].
MONITORING
- Monitor liver function tests as dose reduction may be required in hepatic impairment [1].
- Appropriate clinical and laboratory monitoring (especially of full blood count) are advised if administration of metronidazole for more than 10 days is considered necessary [2,5].
ADVERSE EFFECTS
Gastro-intestinal disturbances (including nausea and vomiting), taste disturbances, furred tongue, oral mucositis, anorexia; very rarely hepatitis, jaundice, pancreatitis, drowsiness, dizziness, headache, ataxia, psychotic disorders, darkening of urine, thrombocytopenia, pancytopenia, myalgia, arthralgia, visual disturbances, rash, pruritus, and erythema multiforme; on prolonged or intensive therapy peripheral neuropathy, transient epileptiform seizures, and leucopenia; also reported aseptic meningitis, optic neuropathy [1]. Anaphylaxis reported rarely [2].
REFERENCES
- British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health, et al. BNF for Children. London: BMJ Group, Pharmaceutical Press and RCPCH Publications Limited; 2020. Available from www.medicinescomplete.com , accessed 24/11/2020.
- B. Braun Melsungen AG. Summary of Product Characteristics for Metronidazole 5mg/ml Solution for Infusion. 2020. Available from www.hpra.ie , accessed 24/11/2020.
- Institute for Safe Medication Practices. ISMP’s List of Confused Drug Names, 2019. Available from www.ismp.org , accessed 24/11/2020.
- UpToDate. Metronidazole (systemic) Paediatric Drug Information: Lexicomp®. Available from www.uptodate.com , accessed 28/11/14.
- Medusa Injectable Drugs Guide. Metronidazole Intravenous – Paediatric Monograph, 2018. Available from www.medusa.wales.nhs.uk , accessed 24/11/2020.
- Department of Health and Government of South Australia. South Australia Neonatal Medication Guidelines – Metronidazole, 2017. Available from http://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/clinical+resources/clinical+topics/neonatal+medication+guidelines , accessed 24/11/2020.
- American Society of Health-System Pharmacists. Pediatric Injectable Drugs. Available from www.medicinescomplete.com , accessed 9/2/15.
- Institute for Safe Medication Practices. FDA and ISMP Lists of Look-Alike Drug Names with Recommended Tall Man Letters, 2016. Available from www.ismp.org , accessed 24/11/2020.
Summary of Changes from Previous Versions
|
Date |
Change |
|
Feb 2021: Rev. No. 1 |
Updated based on Rotunda MetroNIDAZOLE Monograph Jan 2019. Changes to OLOL monograph:
|
|
August 2015 |
This is the first version of this guideline. |
Neonatal - Piperacillin/Tazobactam IV
Neonatal - Piperacillin/Tazobactam IV
Antipseudomonal penicillin with broad spectrum of activity [1].
MEDICATION SAFETY ISSUES
Piperacillin/Tazobactam is a penicillin antibiotic – always check for previous hypersensitivity reactions to penicillins, cephalosporins and other allergens before starting therapy [2].
USES
Piperacillin/Tazobactam is a broad spectrum antibiotic. It is not licensed for use in children less than 2 years, however BNFc provides recommended dosing for neonates if required [1]. It should only prescribed in NICU in OLOL following consultation with clinical microbiologist.
PRESENTATION
Powder for solution for infusion. Each 4.5g vial contains 4g piperacillin (as sodium salt) and 0.5g tazobactam (as sodium salt). Each vial of piperacillin/tazobactam 4.5g contains 9.44 mmol of sodium [2].
DOSAGE [1]
|
Age |
Dose |
Frequency |
|
Neonate |
90mg/kg |
Every 8 hours |
|
Child 1 – 3 months |
90mg/kg |
Every 6 to 8 hours |
Renal Impairment : Dose reduction may be required – refer to BNFc and seek advice [1].
RECONSTITUTION
NB. There are TWO STEPS for reconstitution. BOTH Step 1 and Step 2 below must be followed.
Step 1 : Reconstitute 4.5g vial as per table below using the brand specific displacement value and shake until dissolved [2].
|
Brand |
Displacement Volume [2] |
Diluent to be added |
Final Volume |
Final Concentration |
|
Piperacillin/Tazobactam 4.5g Piperin® Rowex/Sandoz |
3.2mL |
16.8mL WFI or NS |
20mL |
225mg/mL |
Step 2 : Further dilute 4mL of this solution with 6mL sodium chloride 0.9% or glucose 5% to a final volume of 10mL. The resulting solution contains piperacillin/tazobactam 90mg/mL. [1,3,4]
ADMINISTRATION
Administer by IV infusion over 30 minutes [1-3].
SAMPLE CALCULATION
2.8kg neonate under 7 days. Dose: 90mg/kg every 8 hours = 252mg every 8 hours.
Step 1 : Reconstitute 4.5g vial as per table above to give a concentration of 225mg/mL.
Step 2 : Withdraw 4mL of this solution and further dilute with 6mL sodium chloride 0.9% to a final volume of 10mL. The resulting solution contains 90mg/mL. Withdraw 2.8mL (252mg) and give over 30 minutes.
STORAGE
Store unopened vials at room temperature below 25°C [3,5]. Once reconstituted, use immediately [2].
MONITORING
- Monitor renal function – dose reduction may be required in renal impairment [1].
- Caution - high doses may lead to hypernatraemia owing to the high sodium content of the vials [1].
- Monitor FBC - leucopenia and neutropenia may occur, especially during prolonged therapy [2].
- Periodic liver function tests if treatment prolonged more than 10 days [4,5].
ADVERSE EFFECTS
For all penicillins, common or very common adverse effects include diarrhoea, hypersensitivity, nausea, skin reactions, thrombocytopenia and vomiting [1]. Candida superinfection, insomnia and headache are also common with piperacillin/tazobactam [1,2]. Among the most serious adverse reactions, pseudo-membranous colitis and toxic epidermal necrolysis occur in 1 to 10 patients in 10,000 [2]. Please refer to BNFc or SPC for full information on adverse effects.
REFERENCES
- British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health, et al. BNF for Children. London: BMJ Group, Pharmaceutical Press and RCPCH Publications Limited; 2020. Available from www.medicinescomplete.com , accessed 24/11/2020.
- Rowex Limited. Summary of Product Characteristics for Piperacillin/Tazobactam 4g/0.5g Powder for Solution for Infusion. 2018. Available from www.hpra.ie , accessed 24/11/2020.
- Medusa Injectable Drugs Guide. Piperacillin with Tazobactam Intravenous – Paediatric Monograph, 2019. Available from www.medusa.wales.nhs.uk , accessed 24/11/2020.
- Department of Health and Government of South Australia. South Australia Neonatal Medication Guidelines – Piperacillin + Tazobactam, 2017. Available from https://www.sahealth.sa.gov.au/wps/wcm/connect/86ad90804cd7f531baf6baa496684d9f/Piperacillin-tazobactam_Neo_2_0.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-86ad90804cd7f531baf6baa496684d9f-niRbYA1 ,accessed 24/11/2020.
- Gray A et al. Injectable Drugs Guide. London: Pharmaceutical Press; 2020. Available from www.medicinescomplete.com , accessed 24/11/2020.
Summary of Changes from Previous Versions
|
Date |
Change |
|
Feb 2021 |
This is the first version of this guideline. |
Neonatal - Vancomycin IV
Vancomycin Hydrochloride
Vancomycin is a bactericidal antibiotic with activity against some aerobic and anaerobic bacteria including multi-resistant staphylococci.
MEDICATION SAFETY ISSUES
- Incompatible with Cephalosporins, Phenobarbital and Piperacillin-tazobactam.[1]
- Red man syndrome: flushing and hypotension if administered too quickly.
- Vancomycin exposure may be associated with hearing impairment in neonates.[2] Concomitant use of other ototoxic medication, e.g. furosemide and loop diuretics, should be avoided if possible.
USES
Drug of choice for serious infections caused by methicillin-resistant staphylococci and penicillin-resistant pneumococci.[1]
PRESENTATION
Vancomycin Hydrochloride 500mg Powder for concentrate for solution for infusion.[3]
DOSAGE [4-6 ] :
|
DOSE: 15mg/kg for all ages without renal impairment. |
|||
|
Preterm (<37/40 weeks gestation) |
|||
|
Weight |
≤7 days of age |
>7 days of age |
|
|
<1.2kg |
18 HOURLY |
||
|
1.2-2kg |
12 HOURLY |
||
|
>2kg |
12 HOURLY |
8 HOURLY |
|
|
Term ( > 37/40 weeks gestation) |
|||
|
≤7 days of age |
>7 days of age < 28 days of age |
||
|
12 HOURLY |
8 HOURLY |
||
|
Term >28 days |
|||
|
6 HOURLY |
|||
If renal impairment, contact Consultant Microbiologist or Pharmacy for advice.
RECONSTITUTION
Warning- reconstitution is a two step process
Step 1 The displacement value for Vancomycin Mylan brand 500mg (stocked in OLOL) is 0.3ml. Add 9.7ml of Water for Injection to a 500mg vial and shake gently to dissolve. The resulting solution contains 50mg/ml vancomycin .
Step 2 Further dilute 1ml of the reconstituted (50mg/ml) vancomycin solution with 9ml of compatible fluid (e.g. Sodium chloride 0.9%, Glucose 5%). Ensure adequate mixing by inverting the syringe at least 10 times. The resulting solution contains 5mg/ml of vancomycin .
ADMINISTRATION
Use immediately once reconstituted and diluted. Infuse the prescribed dose over at least 60 minutes.
STORAGE
Do not store above 25 o C. Use immediately once reconstituted.
MONITORING
Record vancomycin levels and response on the Vancomycin Monitoring Form (see Appendix 1).
Sub-therapeutic levels can result in treatment failure or the emergence of drug resistance.
Toxic or high levels of vancomycin can result in nephrotoxicity and/or ototoxicity. Monitor creatinine and urea at least twice weekly for the duration of treatment. If the patient is on additional nephrotoxic medication (e.g. NSAID’s, aciclovir, aminoglycosides, diuretics, omeprazole), monitor renal function more frequently.
|
Indication |
Target Trough Concentration |
|
Uncomplicated infections |
10 to 20 mg/L |
|
Complicated infections: (e.g. Bacteraemia, endocarditis, osteomyelitis, meningitis, necrotising fasciitis and empyema) |
15 to 20 mg/L |
|
Dosing Frequency |
When to take a trough level |
|
18 HOURLY |
Up to ONE HOUR before the 2 nd dose When levels are therapeutic, repeat every 2 days |
|
12 HOURLY |
Up to ONE HOUR before the 3rd or 4th dose When levels are therapeutic, repeat every 2 days |
|
8 HOURLY |
Up to ONE HOUR before 4th or 5th dose When levels are therapeutic, repeat every 2 days |
|
6 HOURLY |
Up to ONE HOUR before 4 th , 5th or 6th dose When levels are therapeutic, repeat every 3 days |
|
In patients with normal renal function, DO NOT withhold the next dose while awaiting the result of the trough level – this may result in the patient being under dosed. If patient has renal impairment, contact Consultant Microbiologist or Pharmacy for advice. |
|
Vancomycin Targets and Timing of Levels:
|
Trough Level |
Management |
Timing of Next Level |
|
Less than 5mg/L |
Preterm & term < 1 month: Increase frequency 18hours˃12hours˃8hours>6 hours. For example 18 hours then increase to 12 hourly. Term >1 month : Increase by 20%. |
Complete 24 hours of new regime before checking next level. Following two consecutive levels within therapeutic range repeat every 2-3 days and monitor renal function.
*Complicated infections: Severe infection, reduced sensitivities, bacteraemia, endocarditis, osteomyelitis, meningitis, necrotising fasciitis and empyema |
|
5-9mg/L |
Increase by 10% |
|
|
10-20mg/L |
No change (unless target level is 15-20mg/L for complicated infections* contact micro/ID) |
|
|
21-24mg/L |
Decrease by 10% & Contact Micro |
|
|
> 25mg/L |
Contact microbiology/ ID or Pharmacy for advice |
SIDE EFFECTS
- Nephrotoxicity and ototoxicity.
- Rash and hypotension (red man syndrome) if given too quickly.
- Phlebitis which can be minimized by slow infusion and dilution of the drug.
SAMPLE CALCULATION
0.86kg neonate 28 weeks corrected gestational age.
Dose: 15mg/kg = 15mg x 0.86kg = 12.9mg
Reconstitute and dilute one 500mg vial of vancomycin following the directions above.
The resulting solution contains 5mg/ml of vancomycin.
5mg in 1ml is equivalent to 1mg in 0.2ml
12.9mg in 2.58ml
Infuse a total volume of 2.58ml at a rate of 2.58ml/hr over 60 minutes.
REFERENCES
- Reuters, T., Neofax A manual of drugs used in neonatal care . Vol. 24. 2011.
- Vella-Brincat, J.W., et al., Are gentamicin and/or vancomycin associated with ototoxicity in the neonate? A retrospective audit. Neonatology, 2011. 100 (2): p. 186-93.
- Limited, H.U. Vancomycin Hydrochloride 500 mg and 1 g Powder for Concentrate for Infusion . 2009; Available from: http://www.medicines.org.uk/emc/medicine/20059/SPC .
- British Medical Association, et al., BNF for Children. Accessed via www.medicinescomplete.com November 13th 2018. 2018, BMJ Group and Pharmaceutical Press: London.
- Pickering, L.K., 2009 Red Book: Report of the Committee on Infectious Diseases . 28th ed ed. 2009: American Academy of Paediatrics.
- uptodate.com. Vancomycin: Pediatric Drug Information . 2018.
Appendix 1: Neonatal Vancomycin Monitoring Form - see hardcopy in NICU folder
Summary of Changes from Previous Versions
|
Date |
Change |
|
Jul 2019 |
Revision 2 of the Rotunda Neonatal Vancomycin Monograph received with permission (approved in the Rotunda in Dec 2018). Changes from previous version:
Additional information included in OLOL monograph:
|
|
June 2017 |
Vancomycin Flynn brand now stocked in OLOL. Displacement value of 500mg vial now 0.4ml. Monograph updated to reflect this. |
|
Jan 2015 |
This is the first version of this guideline. It is based on the Rotunda Hospital Neonatal Monograph for Vancomycin, Doc. No. 1, Revision No. 0, date of issue 10/11/14. Changes in OLOL monograph compared to the Rotunda monograph:
|
Neonatal - Varicella Zoster Immunoglobulin (VZIG) IV
Neonatal - Varicella Zoster Immunoglobulin IV (Varitect® CP)
Human varicella-zoster immunoglobulin (VZIG) for intravenous use [1].
MEDICATION SAFETY ISSUES
- This monograph relates to VZIG for intravenous use (Varitect® CP) – the dose and administration of VZIG for intramuscular use is different, please check the product carefully.
- Varicella zoster immunoglobulin may be confused with varicella zoster live vaccine (Varivax®) [2].
USES
See NIAC Guidelines, Chapter on Varicella zoster, for definition of significant exposure to Varicella zoster virus and for full information on use of VZIG for post-exposure prophylaxis in neonates [3].
( https://www.rcpi.ie/Healthcare-Leadership/NIAC/Immunisation-Guidelines-for-Ireland ).
VZIG is recommended for the following groups of neonates following significant exposure to varicella:
- Neonates who are exposed to varicella in mother from 7 days before to 7 days after delivery. Approximately half of these infants may develop varicella despite immunoprophylaxis, but the disease is usually modified. IV aciclovir treatment may occasionally be required. These neonates must receive VZIG as early as possible in the incubation period, as neonatal mortality without VZIG is up to 30%.
-
VZ antibody-negative infants
- exposed to varicella or zoster (other than in the mother) in the first 7 days of life.
- of any age, exposed to varicella or zoster while requiring intensive or prolonged special care.
The NIAC Guidelines advise that the following infants may not have maternal antibodies despite a positive maternal history of varicella and should be tested to determine their VZ antibody status in the event of significant exposure to VZV – in the OLOL NICU, unless urgent testing can be performed, these infants should be given VZIG as soon as possible following discussion with parents:
- born at less than 28 weeks gestation
- weigh less than 1000g at birth
- infants 60 days of age or more still requiring intensive or prolonged special care nursing
- had repeated packed red cell infusions.
The NIAC Guidelines advise that immunocompromised contacts should be tested to determine their VZ antibody status in the event of significant exposure to VZV regardless of the history of varicella – in the OLOL NICU, unless urgent testing can be performed, these infants should be given VZIG as soon as possible following discussion with parents:
- Infants with immunodeficiency syndromes
- Infants receiving steroids
- Infants born to mothers receiving immunosuppressive treatment or therapeutic steroids (NOT including routine ante-natal steroids).
Other infants whose mothers have a positive history of varicella and/or VZV antibodies will usually have maternal antibodies and do not require VZIG.
VZIG is not indicated for full-term infants exposed to VZV (either varicella or zoster) more than 7 days after delivery or if exposure was more than 48 hours before onset of varicella or zoster rash in the index case.
People receiving monthly high-dose IV Human Normal Immunoglobulin (HNIG) are likely to be protected and may not need VZIG if they received the last dose of HNIG within three weeks before exposure.
PRESENTATION
Varitect® CP 25 units/mL solution for intravenous infusion [1].
DOSAGE [1,3]
|
Age |
Dose |
Frequency |
|
Neonate |
1mL/kg (25 units/kg) |
STAT |
ADMINISTRATION [1,3]
- Give by intravenous infusion at an initial rate of 0.1mL/kg/hour for 10 minutes.
- If well tolerated, the rate of administration may be gradually increased to a maximum of 1mL/kg/hour.
- Observe patient for infusion-related reactions during the infusion and for 1 hour after infusion.
SAMPLE CALCULATION
Newborn 3.2kg neonate exposed to varicella in the mother within 7 days before delivery.
Dose: 1mL/kg = 3.2mL. Give by IV infusion at initial rate of 0.32mL/hour for 10 minutes. If well tolerated, gradually increase rate of IV infusion to a maximum of 3.2mL/hour.
STORAGE [1]
Keep container in the outer carton. Store in a refrigerator between 2 o C and 8 o C.
MONITORING [1]
- Observe patient for infusion-related reactions – in the case of adverse reaction, either reduce the rate of administration or stop the infusion, depending on severity.
- Cases of acute renal failure have been reported in patients receiving IVIG therapy. Ensure adequate hydration prior to IVIG initiation, monitor renal function and avoid concomitant use of loop diuretics.
ADVERSE EFFECTS [1,3]
- N.B. - The efficacy of live virus vaccines may be impaired for up to 3 months.
- Adverse reactions such as chills, headache, fever, vomiting, allergic reactions, nausea, arthralgia, low blood pressure and moderate low back pain may occur occasionally.
- Rarely, a sudden fall in blood pressure and, in isolated cases, anaphylactic shock.
- Cases of reversible aseptic meningitis, isolated cases of reversible haemolytic anaemia/haemolysis and rare cases of transient cutaneous reactions, have been observed.
- Increase in serum creatinine level and/or acute renal failure have been observed.
- Very rare cases of thromboembolic reactions such as myocardial infarction, stroke, pulmonary embolism, deep vein thromboses have been observed.
- Cases of Transfusion Related Acute Lung Injury (TRALI).
REFERENCES
- Biotest Pharma. Summary of Product Characteristics for Varitect® CP, October 2019. On file in Pharmacy Department, OLOL.
- Varicella zoster immune globulin (human): Drug information. Available from www.uptodate.com , accessed 19/9/24.
- National Immunisation Advisory Committee. Immunisation Guidelines for Ireland, Chapter 23, Varicella Zoster, updated Oct 2022. Available from https://www.rcpi.ie/Healthcare-Leadership/NIAC/Immunisation-Guidelines-for-Ireland .
Neonatal Antimicrobial Prescribing Principles

Additional Prescribing Principles
-
The ideal antibiotic is effective, safe and cost-effective, does not induce resistance or cause healthcare-associated infection.
-
When prescribing for neonates, gestational age, weight, renal function, hepatic function and concomitant disease should be considered.
-
The prescription of broad spectrum antimicrobials should be reviewed as soon as possible and switched to specific/targeted therapy when culture and sensitivity results become available.
-
Targeted therapy should be used in preference to broad spectrum antimicrobials unless there is a clear clinical reason, for example, mixed infections or life-threatening sepsis. Widespread use of broad spectrum antimicrobials will increase local persistence of resistant organisms, favour opportunistic organisms and often increase cost.
-
Antimicrobial therapy started in other hospitals should be reviewed on admission.
-
High dose therapy for a shorter period is more effective than low dose therapy for a protracted course.
-
The ongoing need for antimicrobial therapy should be reviewed and discussed daily.
-
The development of fungal infections as an adverse effect of antimicrobials is of particular concern in neonates.
-
All serious adverse drug reactions (ADR) should be reported to the Health Products Regulatory Authority (HPRA), previously known as the Irish Medicines Board.The ADR should be reported electronically on www.hpra.ie . The reported ADR and any resulting correspondence should also be recorded in the patient’s medical notes.
-
The use of topical antimicrobial agents should be discouraged as this encourages development of resistance.
-
Prescribe generically unless specifically indicated in these guidelines.
-
See relevant section on antibiotics that are subject to restricted use
-
This guideline should be made available to locum and agency prescribing staff as well as permanent staff.
-
Any deviation in the use of antimicrobials from this guideline should be clearly documented in the patient’s medical record.
Neonatal Empiric Treatment Guidelines
|
Infection |
|
Neonatal - Invasive Candida Infection |
|
Likely Organisms |
|
Candida albicans or other Candida species |
|
Empiric Antimicrobial Treatment |
|
AmBisome® Medication Safety: AmBisome® should be prescribed by brand name only. No other amphotericin product other than AmBisome® should be used. |
|
Comments |
|
|
Duration of Treatment |
|
Minimum 14 days, discuss with Consultant Microbiologist |
|
Infection |
|
Neonatal - Conjunctivitis |
|
Likely Organisms |
|
Mild: S. aureus, S. pneumoniae, H. influenzae Severe: S. aureus, Chlamydia trachomatis, Neisseria gonorrhoeae |
|
Empiric Antimicrobial Treatment |
|
Mild cases: Topical chloramphenicol eye drops, continue for 48 hours after healing. Note - the previous warning associated with use of chloramphenicol eye drops in patients under 2 years of age has been reviewed and removed. Severe cases/purulent eye discharge: Cef-O-taxime IV STAT AND Azithromycin PO 20mg/kg/day, 1 dose daily for 3 days (unlicensed indication) AND Azithromycin eye drops, apply twice daily for 3 days AND If signs of sepsis, ADD Amoxicillin AND Gentamicin |
|
Comments |
|
|
Duration of Treatment |
|
See above. |
|
Infection |
|
Neonatal - Meningitis |
|
Likely Organisms |
|
Group B Streptococci, E. coli, Listeria spp., other Gram-negatives, S. pneumoniae, Enterococcus spp., other Gram-positives |
|
Empiric Antimicrobial Treatment |
|
NICU Setting: See guidelines for early-onset sepsis or late-onset sepsis as indicated Community-Acquired Setting (applicable to ED/5 th Floor): See guidelines for Paediatrics - community acquired sepsis in babies < 8 weeks old |
|
Comments |
|
|
Duration of Treatment |
|
Duration of treatment depends on causative organism:
|
|
Infection |
|
Neonatal - Necrotising Enterocolitis (NEC) |
|
Likely Organisms |
|
Multi-factorial condition, staphylococci, Clostridium perfringens, Klebsiella spp. |
|
Comments |
|
N.B. Always review empiric therapy after 48 hours in conjunction with C&S results. |
|
Empiric Antimicrobial Treatment |
|
Amoxicillin IV AND Gentamicin IV AND Metronidazole Alternative therapy if prolonged treatment course (> 5 days) or renal insufficiency: Amoxicillin IV AND Cef-O-taxime IV AND Metronidazole |
|
Duration of Treatment |
|
Minimum 10 – 14 days |
|
Infection |
|
Neonatal Sepsis Early Onset: within the first 72 hours of life |
|
Likely Organisms |
|
Group B streptococci, E. coli, Listeria monocytogenes, Klebsiella spp., Enterobacter spp., Enterococcus spp., Staphylococcus aureus (uncommon) |
|
Empiric Antimicrobial Treatment |
|
First line : Benzylpenicillin IV AND Gentamicin IV If Listeria suspected, change Benzylpenicillin IV to Amoxicillin IV If meningitis suspected, add Cef-O-taxime IV If baby still unwell or otherwise indicated, consider Aciclovir IV for possible viral infection, discuss with Consultant Neonatologist |
|
Comments |
|
|
Duration of Treatment |
|
Duration of treatment for suspected neonatal sepsis with negative blood cultures:
Duration of treatment for neonatal sepsis with positive blood cultures:
|
|
Infection |
|
Neonatal Sepsis Late Onset: developing after the first 72 hours of life in an infant who is hospitalised |
|
Likely Organisms |
|
Group B streptococci, E. coli, Listeria monocytogenes, Klebsiella spp., Enterobacter spp., Enterococcus spp., Staphylococcus aureus (uncommon), H. influenzae, S. epidermidis, other coagulase-negative staphylococci |
|
Empiric Antimicrobial Treatment |
|
Flucloxacillin IV AND Gentamicin IV If baby is MRSA colonised, replace Flucloxacillin IV with Vancomycin IV If meningitis suspected / if baby very unwell, add Cef-O-taxime IV |
|
Comments |
|
|
Duration of Treatment |
|
Duration of treatment for suspected neonatal sepsis with negative blood cultures:
Duration of treatment for neonatal sepsis with positive blood cultures:
|
|
Infection |
|
Neonatal sepsis: Community-Acquired (applicable to ED/5 th Floor) |
|
Likely Organisms |
|
Group B streptococci, E. coli, Listeria moncytogenes, S. pneumoniae, Klebsiella spp., Enterobacter spp., Enterococcus spp., H. influenzae |
|
Empiric Antimicrobial Treatment |
|
See guidelines for Paediatrics - community acquired sepsis in babies < 8 weeks old |
|
Comments |
|
|
Duration of Treatment |
|
Duration of treatment for suspected neonatal sepsis with negative blood cultures:
Duration of treatment for neonatal sepsis with positive blood cultures:
|
|
Infection |
|
Neonatal - Septic Arthritis and Osteomyelitis |
|
Likely Organisms |
|
Group B streptococci, S. aureus, E. coli |
|
Empiric Antimicrobial Treatment |
|
Flucloxacillin IV AND Cef-O-taxime IV |
|
Comments |
|
N.B. Always review empiric therapy after 48 hours in conjunction with C&S results. |
|
Duration of Treatment |
|
Prolonged duration required, discuss with Consultant Microbiologist. |
|
Infection |
|
Neonatal - Skin Infections |
|
Likely Organisms |
|
Superficial infections: Candida albicans Impetigo and cellulitis: Staphylococcus aureus, streptococci |
|
Empiric Antimicrobial Treatment |
|
If superficial candida infection suspected (i.e. nappy area): Topical miconazole or clotrimazole Impetigo or cellulitis: Flucloxacillin IV AND Benzylpenicillin IV |
|
Comments |
|
|
Duration of Treatment |
|
7 days |
|
Infection |
|
Neonatal - Umbilical Infections |
|
Likely Organisms |
|
Staphylococcus aureus, Group A streptococci, Group B streptococci, E. coli, other gram negative bacilli |
|
Empiric Antimicrobial Treatment |
|
Mild infection with no local “flare” or erythema and no systemic signs: Clean area Moderate Infection: Flucloxacillin IV AND Gentamicin IV Severe Infection / Necrotising Fasciitis: Vancomycin IV AND Gentamicin IV AND Clindamycin IV N.B. Contact Consultant Neonatologist prior to prescribing Clindamycin for neonate |
|
Comments |
|
|
Duration of Treatment |
|
7 - 10 days for moderate infection, duration depends on response/ongoing need for debridement for necrotising fasciitis. |
|
Infection |
|
Neonatal - Urinary Tract Infection |
|
Likely Organisms |
|
E. coli, Klebsiella spp. |
|
Empiric Antimicrobial Treatment |
|
See guidelines for Paediatrics - community acquired sepsis in babies < 8 weeks old |
|
Comments |
|
N.B. Always review empiric therapy after 36 to 48 hours in conjunction with C&S results. |
|
Duration of Treatment |
|
7 to 10 days Avoid prolonged courses of gentamicin (> 5 days) – discuss with Consultant Microbiologist if required. |
|
Infection |
|
Neonatal - Viral Infections |
|
Likely Organisms |
|
Herpes simplex, Varicella zoster, Cytomegalovirus |
|
Empiric Antimicrobial Treatment |
|
Herpes simplex: Aciclovir IV Varicella zoster: Aciclovir IV Congenital cytomegalovirus: Ganciclovir IV - NB. Cytotoxic precautions required. |
|
Comments |
|
N.B. Ganciclovir is mutagenic – cytotoxic precautions required for administration. Contact Pharmacy as soon as possible to order Ganciclovir from aseptic manufacturer. |
|
Duration of Treatment |
|
Herpes simplex: 14 days (at least 21 days if CNS involvement – confirm CSF negative for herpes simplex virus before stopping treatment) Varicella zoster: at least 7 days (given for 10 – 14 days in encephalitis, possibly longer if also immunocompromised) |
Neonatal Medical Prophylaxis
Prevention of Perinatal Transmission of HIV, HBV, HCV and Syphilis
See Rainbow Clinic Guidelines 2015 on the Neonatal Unit, maternity wards and on the hospital HCI-Knowledge .
Download - Rainbow Clinic Guidelines
The Guideline contains detailed information on administration of:
- Hepatitis B vaccines (Energix B Paediatric® and HBvaxPro®)
- Hepatitis B Immunoglobulin (HepatectCP®)
Respiratory Syncytial Virus Prophylaxis
Palivizumab 15mg/kg IM monthly during the RSV season (October to March).
See Standard Operating Procedure Referral Process for Synagis (Palivizumab) administration in the community and administration of Synagis in the Neonatal Intensive Care Unit in Our Lady of Lourdes Hospital Drogheda Nov. 2020, available on the hospital HCI-Knowledge.
Neonatal - Urinary Tract Infection Prophylaxis
Indicated if ultrasound evidence of renal dilatation > 1cm
Likely organisms include E. coli and Klebsiella spp.
Trimethoprim PO is the first line empiric agent for urinary tract infection prophylaxis.
Note that Trimethoprim is not licensed for use in infants under 6 weeks of age.
N.B. Always review empiric therapy in conjunction with C&S results.
Routine Childhood Vaccination Schedule
See www.immunisation.ie for any updated information. The table below outlines the HSE Primary Childhood Immunisation Schedule for babies born after 1 st October 2016. Vaccines should be given at chronological age not corrected gestational age.
|
Vaccine Short Name |
Vaccine Full Name |
|
BCG |
Bacille Calmette Guerin |
|
6 in 1 |
Diphtheria, tetanus, pertussis (acellular), hepatitis B, poliomyelitis (inactivated) and Haemophilus influenzae type b conjugated |
|
PCV |
Pneumococcal polysaccharide conjugate vaccine |
|
Men B |
Meningococcal group B |
|
Rotavirus |
Rotavirus ORAL ( live vaccine ) |
|
Men C |
Meningococcal group C conjugate |
|
MMR |
Measles, mumps and rubella ( live vaccine ) |
|
Hib/Men C |
Haemophilus influenzae type b / meningococcal group C conjugate |
|
Age |
Recommended Vaccine |
Brand Name |
Comment |
|
Birth |
BCG |
BCG |
1 injection |
|
2 months |
6 in 1 + PCV + Men B + Rotavirus |
Infanrix Hexa® + Prevenar 13® + Bexsero® + Rotarix® ORAL |
3 injections and oral drops |
|
4 months |
6 in 1 + Men B + Rotavirus |
Infanrix Hexa® + Bexsero® + Rotarix® ORAL |
2 injections and oral drops |
|
6 months |
6 in 1 + PCV + Men C |
Infanrix Hexa® + Prevenar 13® + Menjugate® |
3 injections |
|
12 months |
MMR + Men B |
Priorix® or MMRVaxpro® + Bexsero® |
2 injections |
|
13 months |
Hib/Men C + PCV |
Menitorix® + Prevenar 13® |
2 injections |
References
References for the Neonatal IV Antimicrobial Monographs are listed at the end of each monograph.
References for all other sections of the neonatal antimicrobial guidelines:
- Mosby's Medical Dictionary, 8th edition. © 2009, Elsevier.Available from http://medical-dictionary.thefreedictionary.com , accessed 08/12/14.
- RCPI Hospital Antimicrobial Stewardship Working Group 2012. Start Smart then Focus Antibiotic Care Bundle.Available from www.rcpi.ie .
- NICE Guideline CG 149. Antibiotics for early-onset neonatal infection: Antibiotics for the prevention and treatment of early-onset neonatal infection. 2012. Available from www.nice.org.uk .
- BMJ Group and Pharmaceutical Press. BNF for Children online.Available from www.medicinescomplete.com , accessed 15/08/18.
- Rotunda Hospital Antimicrobial Guidelines.Neonatal Infections.Accessed via RCSI Hospitals Antimicrobial Guidelines Smartphone Application, 15/08/18.
- Sanford Guide to Antimicrobial Therapy, available from webedition.sanfordguides.com , accessed 15/08/18.
- John Hopkins ABX Guide, available from www.hopkinsguides.com , accessed 15/08/18.
- Scientific Advisory Committee, HPSC. Guidelines for the Early Clinical and Public Health Management of Bacterial Meningitis, 2012, revised 2016. Available from www.hpsc.ie .
- Health Service Executive. Routine Childhood Immunisation Schedule 2016. Available from www.immunisation.ie , accessed 15/08/18.

