Adult Treatment Guidelines
Bone and Joint Infections
|
Indication |
|
Discitis / Vertebral Osteomyelitis / Vertebral Abscess |
|
First Line Antimicrobials |
|
Empiric treatment will vary depending on history and risk factors Discuss all suspected cases with Clinical Microbiology / Infectious Diseases teams. |
|
Comments |
|
|
Duration of Treatment |
|
Expect 6 weeks - ultimate duration depends on individual patient and clinical response. |
|
Indication |
|
Osteomyelitis – Acute |
|
First Line Antimicrobials |
|
Flucloxacillin 2g QDS IV If history of MRSA colonisation, SUBSTITUTE vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. IV to PO switch: Contact Clinical Microbiologist or ID Consultant for advice. |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-AZ-olin 2g TDS IV If history of MRSA colonisation, SUBSTITUTE vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for c alculator and guideline. IV to PO switch: Contact Clinical Microbiologist or ID Consultant for advice. |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. IV to PO switch: Contact Clinical Microbiologist or ID Consultant for advice. |
|
Comments |
|
ALWAYS:
Check before contacting Micro or ID:
Microbiological Investigations:
|
|
Duration of Treatment |
|
Prolonged course of several weeks usually required. Ultimate duration depends on causative pathogen, clinical response, successful source control, blood culture results and absence of other deep foci of infection. |
|
Indication |
|
Osteomyelitis – Chronic |
|
First Line Antimicrobials |
|
The antimicrobial treatment of ALL cases of chronic osteomyelitis should be discussed with Clinical Microbiologist or ID Consultant. Bone specimen should be obtained for culture and sensitivity prior to initiation of antimicrobials – use susceptibilities to guide choice. |
|
Comments |
|
ALWAYS:
Check before contacting Micro or ID:
Microbiological Investigations:
Multidisciplinary management required. The drainage of infection and complete debridement of necrotic bone cannot be overemphasised and is often required for cure. It is not always feasible; however, when possible, debridement enhances the chance of a good outcome and reduces the risk of relapse. |
|
Duration of Treatment |
|
Ultimate duration depends on causative pathogen, clinical response, successful surgical debridement, orthopaedic surgical management plan and blood culture results. |
|
Indication |
|
Septic Arthritis – Native Joint |
|
First Line Antimicrobials |
|
Flucloxacillin 2g QDS IV If history of MRSA colonisation, SUBSTITUTE vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. IV to PO switch: Contact Clinical Microbiologist or ID Consultant for advice. |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-AZ-olin 2g TDS IV If history of MRSA colonisation, SUBSTITUTE vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. IV to PO switch: Contact Clinical Microbiologist or ID Consultant for advice. |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. IV to PO Switch: Contact Clinical Microbiologist or ID Consultant for advice. |
|
Comments |
|
ALWAYS:
Check before contacting Micro or ID:
Microbiological Investigations:
|
|
Duration of Treatment |
|
Minimum 14 days IV followed by PO switch. Ultimate duration depends on causative pathogen, clinical response, successful source control, blood culture results and absence of other deep foci of infection. |
|
Indication |
|
Septic Arthritis – Prosthetic Joint |
|
First Line Antimicrobials |
|
Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. N.B. Empiric rifampicin is not recommended. |
|
Comments |
|
ALWAYS:
Check before contacting Micro or ID:
Microbiological Investigations:
|
|
Duration of Treatment |
|
Ultimate duration depends on causative pathogen, clinical response, successful source control, orthopaedic surgical management plan, blood culture results and absence of other deep foci of infection. |
Candidiasis - Mucocutaneous
|
Indication |
|
Oesophageal Candidiasis |
|
First Line Antimicrobials |
|
Fluconazole 200mg once daily PO If patient not tolerating PO, may use fluconazole 200mg once daily IV |
|
Comments |
|
Microbiological Investigations:
Send HIV serology in patients not known to be immunocompromised. Discuss with Clinical Microbiologist if patient has severe oesophageal candidiasis or is severely immunocompromised – higher doses and longer duration may be indicated. Monitor liver function tests while on fluconazole. |
|
Duration of Treatment |
|
14 days |
|
Indication |
|
Oral Candidiasis |
|
First Line Antimicrobials |
|
Mild: Nystatin oral suspension 1 – 6ml QDS PO OR Miconazole gel 2.5ml QDS PO after meals
Patients administered nystatin / miconazole need to be able to swish suspension in mouth / keep the gel in contact with the affected areas for as long as possible and then swallow.
Moderate to Severe: Fluconazole 200mg daily PO |
|
Comments |
|
Microbiological Investigations:
|
|
Duration of Treatment |
|
7 to 14 days. Nystatin: Continue for at least 48 hours after symptoms have disappeared (re-assess if required for more than 14 days). Miconazole: Continue for 1 week after symptoms have disappeared. |
|
Indication |
|
Vulvovaginal Candidiasis |
|
First Line Antimicrobials |
|
Acute vulvovaginal candidiasis: Clotrimazole 500mg pessary PV STAT OR Clotrimazole 2% cream topically BD or TDS OR Fluconazole 150mg PO STAT Recurrent vulvovaginal candidiasis: Refer patient to GU/ID service for confirmation of diagnosis and further management. Pregnant patient: Please check LH Obstetrics and Gynaecology Guidelines for management. |
|
Comments |
|
Microbiological Investigations:
|
|
Duration of Treatment |
|
Cardiovascular Infections
|
Indication |
|
Infective Endocarditis - Community-Acquired Native Valve or Late Prosthetic Valve (> 12 months post-surgery) Endocarditis |
|
First Line Antimicrobials |
|
Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. AND Gentamicin 3mg/kg once daily IV (note lower than usual dose) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
ALWAYS:
Echocardiography required (TTE +/- TOE) – discuss with Cardiology. Consider early review by cardiothoracic surgery - discuss with Cardiology. Microbiological Investigations:
|
|
Duration of Treatment |
|
Ultimate duration depends on causative pathogen, clinical response, blood culture results and type of infected valve. |
|
Indication |
|
Infective Endocarditis - Early Prosthetic Valve Endocarditis (< 12 months post-surgery) or Healthcare-Associated Endocarditis |
|
First Line Antimicrobials |
|
Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. AND Gentamicin 3mg/kg once daily IV (note lower than usual dose) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
ALWAYS:
Echocardiography required (TTE +/- TOE) – discuss with Cardiology. Consider early review by cardiothoracic surgery - discuss with Cardiology. Microbiological Investigations:
|
|
Duration of Treatment |
|
Ultimate duration depends on causative pathogen, clinical response, blood culture results and type of infected valve. |
Central Nervous System Infections
|
Indication |
|
Encephalitis |
|
First Line Antimicrobials |
|
Aciclovir 10mg/kg TDS IV N.B. Adjust dose if renal impairment . N.B. For obese patients (BMI > 30kg/m 2 ), use of obese-dosing weight (ODW) is recommended. Calculate obese-dosing weight (ODW)
Use of actual body weight can lead to toxicity. Use of ideal body weight can result in under-dosing. Take severity of infection and renal function into account when choosing dose and monitor patient for nephrotoxicity or neurotoxicity when high doses are used. |
|
Comments |
|
Microbiological Investigations:
Public Health notification required for viral encephalitis. |
|
Duration of Treatment |
|
14 to 21 days- Discuss all cases with Clinical Microbiology or Infectious Diseases teams |
|
Indication |
|
Meningitis |
|
First Line Antimicrobials |
|
Cef-TRI-axone 2g BD IV AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. AND If Listeria meningitis suspected, ADD Amoxicillin 2g four hourly IV Risk factors for Listeria spp . include age > 65 years, immunocompromised, pregnant. AND Dexamethasone phosphate 0.15mg/kg QDS IV (maximum 10mg per dose) started before or with first dose of antimicrobial therapy and continued for 4 days. |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-TRI-axone 2g BD IV AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. AND If Listeria meningitis suspected, additional cover required:
Risk factors for Listeria spp. include age > 65 years, immunocompromised, pregnant AND Dexamethasone phosphate 0.15mg/kg QDS IV (maximum 10mg per dose) started before or with first dose of antimicrobial therapy and continued for 4 days. |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Meropenem 2g TDS IV N.B. Use meropenem with caution and close clinical monitoring if history of immediate-onset penicillin hypersensitivity - approximately 1% risk of immediate-onset hypersensitivity reaction to meropenem. AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV (Do not load pregnant patients) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. AND Dexamethasone phosphate 0.15mg/kg QDS IV (maximum 10mg per dose) started before or with first dose of antimicrobial therapy and continued for 4 days. This regimen covers Listeria spp. |
|
Comments |
|
ALWAYS:
Microbiological Investigations:
Public Health notification required for meningitis caused by N. meningitidis, H. influenzae, S. pneumoniae, Listeria spp. and viral meningitis. N.B. See chemoprophylaxis for meningococcal contacts . |
|
Duration of Treatment |
|
Duration depends on causative organism:
|
Ear Nose and Throat
|
Centor Score |
Estimated Risk of GAS Pharyngitis |
|
Score one point for each sign present:
|
0 signs = 2.5% risk of GAS pharyngitis 1 sign = 6.5% risk of GAS pharyngitis 2 signs = 15% risk of GAS pharyngitis 3 signs = 32% risk of GAS pharyngitis 4 signs = 56% risk of GAS pharyngitis |
|
Acute Sore Throat |
|
First Line Antimicrobials |
|
Mostly viral. Centor Score 3-4: Modest benefit of antimicrobials in symptom reduction. Watchful waiting/delayed prescription strategy (patient waits for 1-2 days and fills prescription if still not better by then or GAS cultured from swab) is a valid option. Phenoxymethylpenicillin (Calvepen®) 666mg QDS PO If unable to tolerate oral medications: Benzylpenicillin 1.2g QDS IV |
|
Penicillin Hypersensitivity |
|
As above, if antimicrobials indicated: Clarithromycin 500mg BD PO N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval |
|
Comments |
|
Sore throat should not be treated with antimicrobials to prevent development of rheumatic fever and acute glomerulonephritis in low-risk patients. The prevention of suppurative complications (quinsy, otitis media, cervical lymphadenitis, acute otitis media, mastoiditis or sinusitis) is not a specific indication for antimicrobial therapy in acute sore throat.
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations:
Consider Corynebacterium diphtheriae – Droplet transmission, gradual onset sore throat, fever, exudative pharyngitis, which may progress within 3 days to thick grey pseudomembranes firmly attached to underlying mucosa – up to 20% mortality secondary to toxin effects – Check vaccination history and inform on-call Clinical Microbiologist immediately if suspected diphtheria http://www.hpsc.ie/A-Z/VaccinePreventable/Diphtheria/
Consider EBV infection in young adult with pharyngitis, fever, cervical adenopathy
|
|
Duration of Treatment |
|
10 days |
|
Indication |
|
Peritonsillar Abscess (Quinsy)
|
|
First Line Antimicrobials |
|
Co-amoxiclav 1.2gm TDS IV
Empiric IV to PO switch: Co-amoxiclav 625mg TDS PO |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND
Empiric IV to PO switch: Cefaclor LA 750mg BD PO AND Metronidazole 400mg TDS PO |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Clindamycin 450mg QDS PO or 600mg QDS IV (excellent oral bioavailability) |
|
Comments |
|
N.B. The primary treatment of an abscess is surgical drainage.
Risk factors for peritonsillar abscess include smoking, poor periodontal hygiene, male gender, prior antimicrobials, immunocompromise and ages 15 – 40 years.
Lemierre’s syndrome is a rare complication arising after pharyngitis due to Fusobacterium necrophorum:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations:
|
|
Duration of Treatment |
|
7 to 10 days. Ultimate duration dictated by clinical response and adequate source control (i.e. drainage of abscess). |
|
Indication |
|
Perichondritis Infection of the perichondrium layer surrounding the outer ear cartilage, often associated with trauma – e.g. piercing. Insidious onset. |
|
First Line Antimicrobials |
|
Flucloxacillin 2g QDS IV AND Ciprofloxacin 500mg BD PO (or 400mg BD IV only where oral route is not feasible - excellent oral bioavailability) N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered.
Empiric IV to PO switch: Flucloxacillin 1g QDS PO AND Ciprofloxacin 500mg BD PO |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Ciprofloxacin 500mg BD PO (or 400mg BD IV only where oral route is not feasible - excellent oral bioavailability) N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered.
Empiric IV to PO switch: Cef-AL-exin 500mg TDS PO AND Ciprofloxacin 500mg BD PO |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Clindamycin 450mg QDS PO or 600mg QDS IV (excellent oral bioavailability) AND Ciprofloxacin 500mg BD PO (or 400mg BD IV only where oral route is not feasible - excellent oral bioavailability) N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. |
|
Comments |
|
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations:
|
|
Duration of Treatment |
|
7 days |
|
Indication |
|
Acute Otitis Externa AKA ‘Swimmer’s Ear’ Diffuse inflammation of external ear canal, which may involve pinna or tympanic membrane:
|
|
First Line Antimicrobials |
|
Topical therapy is first choice . High concentration of topical antimicrobial delivered to infected area. If non-intact tympanic membrane or grommets, do not use gentamicin or neomycin preparations (ototoxic).
Kenacomb Otic® (Triamcinolone acetonide, Nystatin, Gramicidin and Neomycin): Apply ointment to affected ear TDS OR Genticin® 0.3% (Gentamicin): Instill 3 drops into affected ear TDS OR Ciloxan® (Ciprofloxacin): Instill 4 drops into affected ear BD
Systemic therapy is second line and preferred option if:
Outpatient Management: Ciprofloxacin 500mg BD PO N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered.
Inpatient Management: Flucloxacillin 2g QDS IV AND Ciprofloxacin 500mg BD PO (or 400mg BD IV only where oral route is not feasible - excellent oral bioavailability) N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered.
Empiric IV to PO switch: Flucloxacillin 1g QDS PO AND Ciprofloxacin 500mg BD PO |
|
Penicillin Hypersensitivity |
|
As above, topical therapy is first line. Systemic therapy choice if required:
Outpatient Management: Ciprofloxacin 500mg BD PO N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered.
Inpatient Management: Clindamycin 450mg QDS PO or 600mg QDS IV (excellent oral bioavailability) AND Ciprofloxacin 500mg BD PO (or 400mg BD IV only where oral route is not feasible - excellent oral bioavailability) N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. |
|
Comments |
|
Prescribe adequate analgesia, aural toilet, consider wick if obstruction present.
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations:
|
|
Duration of Treatment |
|
Topical or systemic therapy: 7 days |
|
Indication |
|
Fungal Otitis Externa AKA Otomycosis Aspergillus spp or Candida spp.
|
|
First Line Antimicrobials |
|
Careful drying and cleaning of external auditory canal, followed by topical therapy: Topical acetic acid – Patient prepares a solution of one part table vinegar plus four parts boiled water – Instill 3 drops into affected ear TDS OR Topical clotrimazole 1% cream
Systemic therapy is second line: Choose topical therapy unless:
|
|
Comments |
|
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations:
|
|
Duration of Treatment |
|
Topical or systemic therapy: 7 days |
|
Indication |
|
Acute Otitis Media Visualisation of the tympanic membrane is essential for diagnosis of acute otitis media. |
|
First Line Antimicrobials |
|
Most cases are viral (RSV, influenza, parainfluenza, adenovirus, rhinovirus and enterovirus) and antimicrobial therapy is not routinely indicated.
Watchful waiting/delayed prescription strategy (patient waits for up to seven days and only fills prescription if still not better by then) is a valid option for patients aged >2 years. However, prescribe antimicrobials immediately if:
[Please refer to LH Paediatric Antimicrobial Guidelines for management of acute otitis media in paediatrics.]
First Line Antimicrobials: Amoxicillin 1g TDS PO OR Clarithromycin 500mg BD PO N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval
Second Line Antimicrobials: Co-amoxiclav 625mg TDS PO |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
As above, if antimicrobials indicated: Clarithromycin 500mg BD PO N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
As above, if antimicrobials indicated: Clarithromycin 500mg BD PO N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval |
|
Comments |
|
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations:
|
|
Duration of Treatment |
|
5 days |
|
Indication |
|
Acute Mastoiditis - Uncomplicated Acute inflammation of the mastoid periostium and air cells. A complication of acute otitis media – Signs of acute otitis media on otoscopy accompanied by otalgia, retroauricular swelling and/or erythema, ear protrusion, mastoid tenderness and otorrhoea. |
|
First Line Antimicrobials |
|
Uncomplicated Infection: Co-amoxiclav 1.2gm TDS IV
Empiric IV to PO switch: Co-amoxiclav 625mg TDS PO |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (or 500mg TDS IV only where oral route is not feasible - excellent oral bioavailability)
Empiric IV to PO switch: Cefaclor LA 750mg BD PO AND Metronidazole 400mg TDS PO |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Levofloxacin 500mg BD PO or IV (excellent oral bioavailability) N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (or 500mg TDS IV only where oral route is not feasible - excellent oral bioavailability) |
|
Comments |
|
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations:
|
|
Duration of Treatment |
|
10-14 days. Ultimate duration will be dictated by clinical response and adequate source control (e.g. adequate drainage). |
|
Indication |
|
Acute Mastoiditis - Complicated Acute mastoiditis is considered complicated if subperiosteal abscess/intracranial extension or cochlear implant in situ. |
|
First Line Antimicrobials |
|
Complicated infection: Cef-TRI-axone 2g BD IV AND Metronidazole 500mg TDS IV |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-TRI-axone 2g BD IV AND Metronidazole 500mg TDS IV |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Contact Clinical Microbiologist for advice. |
|
Comments |
|
If grommets in situ or meningitis suspected in setting of a cochlear implant – ALWAYS discuss with Clinical Microbiologist.
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations:
|
|
Duration of Treatment |
|
Duration will be dictated by clinical response and adequate source control (e.g. adequate drainage). |
|
Indication |
|
Rhinosinusitis: Acute < 4 Weeks Duration Symptomatic inflammation of paranasal sinuses and nasal cavity. Up to four weeks of purulent nasal discharge (anterior and or posterior), nasal obstruction, facial pain, pressure or fullness. |
|
First Line Antimicrobials |
|
Mostly viral (RSV, influenza, parainfluenza, adenovirus, rhinovirus and enterovirus) and antimicrobials are not routinely indicated. Consider bacterial sinusitis where symptoms or signs fail to improve within 10 days or worsen following an initial improvement.
Watchful waiting/delayed prescription strategy (patient waits for up to seven days and only fills prescription if still not better by then) is a valid option.
First Line Antimicrobials: Amoxicillin 500mg TDS PO
Second Line Antimicrobials: Co-amoxiclav 625mg TDS PO |
|
Penicillin Hypersensitivity |
|
As above. If antimicrobials indicated: Doxycycline 200mg once daily PO OR Clarithromycin 500mg BD PO N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval |
|
Comments |
|
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations: Sinus or meatal cultures recommended if:
Nasopharyngeal cultures are unreliable and not recommended. |
|
Duration of Treatment |
|
5 days |
|
Indication |
|
Rhinosinusitis: Chronic >12 Weeks Duration Differentiate between recurrent episodes of acute rhinosinusitis and true chronic rhinosinusitis; persistent symptomatic inflammation of sinonasal cavities lasting longer than three months. Confirm clinical diagnosis, with objective evidence of sinonasal inflammation and record presence or absence of nasal polyps which may influence management. |
|
First Line Antimicrobials |
|
In addition to the maintenance medical therapies, the use of oral antimicrobials may be required in acute exacerbations with superimposed infection. Antifungal therapy is not recommended.
Co-amoxiclav 625mg TDS PO |
|
Penicillin Hypersensitivity |
|
As above. If antimicrobials indicated: Doxycycline 200mg once daily PO OR Clarithromycin 500mg BD PO N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval |
|
Comments |
|
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations: Sinus or meatal cultures recommended if:
Nasopharyngeal cultures are unreliable and not recommended. |
|
Duration of Treatment |
|
14 days |
|
Indication |
|
Sialadenitis: Acute Bacterial Inflammation and enlargement of salivary glands. Patient may present with fever, acute pain, acute facial swelling, usually unilateral and may be worsened on eating/swallowing. Bacterial infection can occur in setting of reduced saliva flow:
|
|
First Line Antimicrobials |
|
Inpatient treatment required if inability to open mouth, cranial nerve involvement or systemically unwell: Co-amoxiclav 1.2gm TDS IV
Empiric IV to PO switch: Co-amoxiclav 625mg TDS PO |
|
Penicillin Hypersensitivity |
|
Clindamycin 450mg QDS PO or 600mg QDS IV (excellent oral bioavailability) |
|
Comments |
|
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations:
|
|
Duration of Treatment |
|
7 days |
|
Indication |
|
Sialadenitis: Viral – Mumps
|
|
First Line Antimicrobials |
|
Antimicrobial therapy not indicated for viral infection. |
|
Comments |
|
If mumps suspected, refer to LH Regional Infection Control Guidelines and inform IPCT: Patient isolation with droplet precautions may be indicated. Patients with mumps are considered infectious for two days prior to and five days after symptom onset.
It is very important to confirm the diagnosis of suspected mumps infection:
Public Health notification is required for mumps infection. |
|
Indication |
|
Dental Abscess
|
|
First Line Antimicrobials |
|
Mild to Moderate Infection: Amoxicillin 500mg-1g TDS PO
Severe Infection: Co-amoxiclav 1.2g TDS IV
Severe infection indicated by:
|
|
Penicillin Hypersensitivity |
|
Mild to Moderate Infection: Metronidazole 400mg TDS PO
Severe infection: Clindamycin 600mg QDS IV N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval |
|
Comments |
|
N.B. The primary treatment of an abscess is surgical drainage.
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours.
Microbiological Investigations:
|
|
Duration of Treatment |
|
5 days |
Gastrointestinal Infections
|
Indication |
|
Acute Gastro-Enteritis |
|
First Line Antimicrobials |
|
Empiric antibiotics are not usually required pending culture results and may be harmful in cases of verocytotoxogenic E. coli (VTEC) infection. |
|
Comments |
|
If infectious diarrhoea is suspected or confirmed, inform Infection Prevention and Control Team (IPCT) and isolate patient with standard and contact precautions. There are many potential underlying causes in a patient presenting with diarrhea – the following mnemonic ( SIGHT ) may be helpful in the initial management of diarrhea of unknown cause: S – Suspect the diarrhea may be due to an infective cause I – Isolate the patient G – Gloves and aprons to be worn by healthcare workers in contact with the patient and his/her environment H – Hand hygiene with soap and water is preferred (alcohol-based hand rubs are not effective against spores of C. difficile ) T – Test for faeces for C . difficile and enteric pathogens that cause infective diarrhoea. Microbiological Investigations:
Public Health notification is required for cases of salmonellosis, shigellosis, campylobacterioisis or VTEC infection. |
|
Indication |
|
Clostridioides difficile Infection (CDI) Severe CDI is associated with any of:
Severe AND complicated CDI implies severe disease with hypotension, shock, rising serum lactate, ileus or toxic megacolon. |
|
First Line Antimicrobials |
|
Mild to Moderate CDI: Vancomycin 125mg QDS PO
Severe CDI: Early surgical opinion Vancomycin 125mg QDS PO
Severe complicated CDI: Early surgical opinion Vancomycin 500mg QDS PO AND Metronidazole 500mg TDS IV |
|
Administration of ORAL vancomycin |
|
For inpatients: Vancomycin injection vials may be given ORALLY or ENTERALLY. Reconstitute vancomycin 500mg vial with 10mL WFI. Take required dose (125mg=2.5mL) and dilute dose further with approx. 30mL water before administration. If applicable, the remainder of the reconstituted vial may be stored in the fridge for up to 24 hours if labelled with the patient’s name (single patient use only).
For patients to be discharged on ORAL vancomycin: Oral vancomycin capsules are available in the community. A Hi-Tech prescription is no longer required. Prior to discharge, please inform patient's community pharmacy in advance to allow time for them to order oral vancomycin. |
| Comments |
|
Always suspect CDI if:
ALWAYS CONTACT Clinical Microbiologist for advice. Patients with CDI should be REVIEWED DAILY for signs of severe infection as above. Contact Clinical Microbiologist again if patient’s symptoms fail to resolve after 48 hours of treatment. If CDI is suspected or confirmed, inform IPCT and isolate patient with standard and contact precautions:
Microbiological Investigations:
If patient has CDI:
If patient has RECURRENT CDI or if fidaxomicin treatment is being considered, please contact Clinical Microbiologist for advice as fidaxomicin is a restricted agent. A Root-Cause-Analysis Investigation will be undertaken for healthcare-associated severe CDI in conjunction with the IPCT. N.B . Oral vancomycin is indicated only for CDI. It is active within the gastrointestinal tract and is not absorbed. It should never be used for systemic treatment. Drug levels are not required. |
|
Duration of Treatment |
|
10 days |
|
Indication |
||
|
Helicobacter pylori Infection |
||
|
First Line Antimicrobials |
||
|
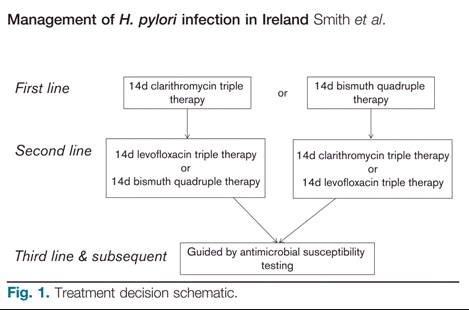
The following treatment algorithm is recommended by the Irish Helicobacter pylori Working Group 2017 – it corresponds to recommendations of the Maastricht V Guidelines 2016:
|
||
|
Treatment Regimen |
Description |
Duration |
|
Clarithromycin-based triple therapy |
|
14 days |
|
Bismuth quadruple therapy |
|
14 days |
|
Levofloxacin-based triple therapy** |
|
14 days |
|
* Tripotassium Dicitratobismuthate is unlicensed in the Republic of Ireland and can be difficult to obtain in community pharmacy. Gastrodenol® (Spanish brand) is currently available from IDIS or Medisource wholesaler (May 2018). Advise patient that all 4 medicines in the regimen must be taken together. Advise community pharmacist (by documenting on the prescription) to contact the hospital pharmacy if difficulty is encountered in obtaining bismuth Rx (OLOL 041 9874663, LCH 042 9385441). |
||
|
**N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. |
||
|
Penicillin Allergy Alternatives |
||
|
N.B. Double check that the patient is truly penicillin allergic as there are fewer treatment options for patients who are allergic to penicillin. Use the bismuth quadruple therapy treatment regimen listed above as the first line regimen. In the case of treatment failure, please contact the Consultant Gastroenterologist and/or Consultant Microbiologist for advice. |
||
|
Comments |
||
|
Non-invasive Investigations:
Invasive Investigations:
Other Comments:
|
||
|
Duration of Treatment |
||
|
14 days for each regimen as listed above. |
||
Genital Tract Infections
|
Indication |
|
Acute Epididymo-orchitis Higher risk of STI-associated epididymo-orchitis?
Lower risk of STI-associated epididymo-orchitis?
|
|
First Line Antimicrobials |
|
STI likely/possible: Doxycycline 100mg BD PO for 14 days AND If N. gonorrhoeae strongly suspected, ADD Cef-TRI-axone 1g IM stat STI unlikely: Ciprofloxacin 500mg BD PO for 10 days N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered . |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
STI likely/possible: Doxycycline 100mg BD PO for 14 days AND If N. gonorrhoeae strongly suspected, ADD Cef-TRI-axone 1g IM stat STI unlikely: Ciprofloxacin 500mg BD PO for 10 days N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered . |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
STI likely/possible: Contact GU/ID Consultant for advice. STI unlikely: Ciprofloxacin 500mg BD PO for 10 days N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. |
|
Comments |
|
ALWAYS take a sexual history, regardless of patient age. Seek urgent urology review if concern regarding testicular torsion. Microbiological Investigations:
NB. Refer patient to GUM clinic if indicated : 086 8241847. |
|
Duration of Treatment |
|
Duration of each agent as listed in the dosing section. |
|
Indication |
|
Acute Prostatitis |
|
First Line Antimicrobials |
|
Ciprofloxacin 500mg BD PO N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered . |
|
Comments |
|
ALWAYS contact Consultant Urologist for advice. Microbiological Investigations:
|
|
Duration of Treatment |
|
Review treatment after 14 days and either stop or continue for a further 14 days if needed (based on history, symptoms, clinical examination, urine and blood tests). |
|
Indication |
|
Pelvic Inflammatory Disease: Mild to Moderate - Outpatient Treatment |
|
First Line Antimicrobials |
|
NOT pregnant or breast-feeding: Doxycycline 100mg BD PO for 14 days AND Metronidazole 400mg BD PO for 14 days AND If N. gonorrhoeae strongly suspected, ADD Cef-TRI-axone 1g IM stat |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
NOT pregnant or breast-feeding: Doxycycline 100mg BD PO for 14 days AND Metronidazole 400mg BD PO for 14 days AND If N. gonorrhoeae strongly suspected, ADD Cef-TRI-axone 1g IM stat |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Contact GU/ID Consultant for advice. |
|
Comments |
|
ALWAYS take a sexual history, regardless of patient age. If intra-uterine contraceptive device (IUCD) in situ , seek GU or Gynaecology advice. Microbiological Investigations:
N.B. Refer patient and partner to the GUM clinic: 086 8241847. |
|
Duration of Treatment |
|
Duration of each agent as listed in the dosing section. |
|
Indication |
|
Pelvic Inflammatory Disease: Severe – Inpatient Treatment |
|
First Line Antimicrobials |
|
NOT pregnant or breast-feeding: Doxycycline 100mg BD PO (if cannot tolerate PO, Erythromycin 500mg QDS IV) AND Metronidazole 400mg BD PO (excellent oral bioavailability) or Metronidazole 500mg BD IV only where oral route is not feasible AND Cef-TRI-axone 2g daily IV Empiric IV to oral switch: Doxycycline 100mg BD PO AND Metronidazole 400mg BD PO to complete 14 days total.
Pregnant patient: ALWAYS seek Gynaecology advice. Erythromycin 500mg QDS PO (if cannot tolerate PO, Erythromycin 500mg QDS IV) AND Metronidazole 400mg BD PO (excellent oral bioavailability) or Metronidazole 500mg BD IV only where oral route is not feasible AND Cef-TRI-axone 2g daily IV Empiric IV to oral switch: Erythromycin 500mg QDS PO AND Metronidazole 400mg BD PO to complete 14 days total.
|
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
NOT pregnant or breast-feeding: Doxycycline 100mg BD PO (if cannot tolerate PO, Erythromycin 500mg QDS IV) AND Metronidazole 400mg BD PO (excellent oral bioavailability) or Metronidazole 500mg BD IV only where oral route is not feasible AND Cef-TRI-axone 2g daily IV Empiric IV to oral switch: Doxycycline 100mg BD PO AND Metronidazole 400mg BD PO to complete 14 days total.
Pregnant patient: ALWAYS seek Gynaecology advice. Erythromycin 500mg QDS PO (if cannot tolerate PO, Erythromycin 500mg QDS IV) AND Metronidazole 400mg BD PO (excellent oral bioavailability) or Metronidazole 500mg BD IV only where oral route is not feasible AND Cef-TRI-axone 2g daily IV Empiric IV to oral switch: Erythromycin 500mg QDS PO AND Metronidazole 400mg BD PO to complete 14 days total. |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Contact GU/ID Consultant for advice. |
|
Comments |
|
ALWAYS take a sexual history, regardless of patient age. If intra-uterine contraceptive device (IUCD) in situ , seek gynaecology advice. Microbiological Investigations:
N.B. Refer patient and partner to the GUM clinic: 086 8241847. |
|
Duration of Treatment |
|
Duration of each agent as listed in the dosing section. |
|
Indication |
|
Scrotal abscess |
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
If Fournier’s gangrene, refer to Necrotising Skin and Soft Tissue Infections N.B. The primary treatment of an abscess is surgical drainage. Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
5 days – ultimate duration depends on clinical response and adequate source control (e.g. adequate drainage). |
For other genital conditions, see HSE Antibiotic Prescribing Guidelines for management.
Head and Neck Infections
|
Indication |
|
Orbital cellulitis |
|
First Line Antimicrobials |
|
Cef-O-taxime 1g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible AND Flucloxacillin 2g QDS IV If history of MRSA colonisation, replace Flucloxacillin with Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. Empiric IV to PO switch: Co-amoxiclav 625mg TDS PO |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-O-taxime 1g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. Empiric IV to PO switch: Cef-AL-exin 500mg TDS PO AND Metronidazole 400mg TDS PO |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. AND Levofloxacin 500mg BD PO or IV (excellent oral bioavailability) N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible Empiric IV to PO switch: Levofloxacin 500mg BD PO AND Metronidazole 400mg TDS PO |
|
Comments |
|
ALWAYS:
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
7 to 14 days depending on clinical response |
Hepatobiliary and Pancreatic Infections
|
Indication |
|
Acute Cholecystitis |
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. Empiric IV to PO switch: Cefaclor LA 750mg BD PO AND Metronidazole 400mg TDS PO |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. Empiric IV to PO switch: Cefaclor LA 750mg BD PO AND Metronidazole 400mg TDS PO |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral roue is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
N.B. Review need for gentamicin daily. Avoid durations in excess of 5 days. Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
5 to 7 days |
|
Indication |
|
Acute Cholangitis |
|
First Line Antimicrobials |
|
Pip/tazobactam 4.5g TDS IV AND Gentamicin 5mg/kg daily IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible AND Gentamicin 5mg/kg daily IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
N.B. Review need for gentamicin daily. Avoid durations in excess of 5 days. Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
7 days - ultimate duration dictated by clinical response, blood culture results and adequate source control (e.g. adequate drainage). |
|
Indication |
|
Acute Pancreatitis Antibiotics are not routinely indicated in pancreatitis. They are indicated for sepsis, necrotising pancreatitis with > 30% necrosis and acute gallstone pancreatitis. If the patient has clinical sepsis, ensure the “Sepsis 6” care bundle is completed within 1 hour. Review indication for ongoing antimicrobials once culture results available. |
|
First Line Antimicrobials |
|
Pip/tazobactam 4.5g TDS IV +/- Gentamicin 5mg/kg daily IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
7 days – ultimate duration dictated by clinical response, blood culture results and adequate source control (e.g. adequate drainage). |
|
Indication |
|
Spontaneous Bacterial Peritonitis |
|
First Line Antimicrobials |
|
Cef-TRI-axone 2g once daily IV |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-TRI-axone 2g once daily IV |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. |
|
Comments |
|
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. Gastroenterology consult recommended. |
|
Duration of Treatment |
|
5 days |
Intra-abdominal Infections
|
Indication |
|
Appendicitis |
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. Empiric IV to PO switch: Cefaclor LA 750mg BD PO AND Metronidazole 400mg TDS PO |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. Empiric IV to PO switch: Cefaclor LA 750mg BD PO AND Metronidazole 400mg TDS PO |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV onlywhere oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
N.B. Review need for gentamicin daily. Avoid duration > 5 days. ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. Microbiological Investigations:
|
|
Duration of Treatment |
|
Uncomplicated appendicitis: Post-operative antimicrobials not indicated. Complicated or perforated appendicitis: 5 to 7 days - ultimate duration dictated by clinical response, blood culture results and adequate source control (e.g. adequate drainage). |
|
Indication |
|
Community-Acquired Intra-Abdominal Infections Includes: Diverticulitis, peritonitis, abscess, GI perforation |
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
N.B. Review need for gentamicin daily. Avoid duration > 5 days. N.B. The primary treatment of an abscess is surgical drainage. Consider addition of antifungal if upper GI perforation, patient immunocompromised or critical care admission. Discuss with Clinical Microbiologist regarding choice of antifungal. ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. Microbiological Investigations:
|
|
Duration of Treatment |
|
5 to 7 days - ultimate duration dictated by clinical response, blood culture results and adequate source control (e.g. adequate drainage). |
|
Indication |
|
Hospital-Acquired Intra-Abdominal Infections Includes: Peritonitis, abscess, GI perforation |
|
First Line Antimicrobials |
|
Pip/tazobactam 4.5g TDS IV +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
N.B. Review need for gentamicin daily. Avoid duration > 5 days. N.B. The primary treatment of an abscess is surgical drainage. Consider addition of antifungal if upper GI perforation, patient immunocompromised or critical care admission. Discuss with Clinical Microbiologist regarding choice of antifungal. Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
5 to 7 days - ultimate duration dictated by clinical response, blood culture results and adequate source control (e.g. adequate drainage). |
|
Indication |
|
Infected Pilonidal Sinus |
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible Empiric IV to PO switch: Cef-AL-exin 500mg TDS PO AND Metronidazole 400mg TDS PO |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible Empiric IV to PO switch: Cef-AL-exin 500mg TDS PO AND Metronidazole 400mg TDS PO |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible |
|
Comments |
|
N.B. The primary treatment of an abscess is drainage. Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
3 to 5 days |
|
Indication |
| Perianal Abscess and Ischiorectal Abscess |
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. Empiric IV to PO switch: Cef-AL-exin 500mg TDS PO AND Metronidazole 400mg TDS PO |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. Empiric IV to PO switch: Cef-AL-exin 500mg TDS PO AND Metronidazole 400mg TDS PO |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible +/- Gentamicin 5mg/kg daily IV (if clinical sepsis) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
N.B. The primary treatment of an abscess is surgical drainage. Microbiological Investigations:
ALWAYS REVIEW empiric antimicrobial therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
5 days |
Malaria
|
Indication |
|
Malaria - Severe > 2% of red blood cells parasitised or end organ damage P. falciparum |
|
Antimalarial Treatment |
|
First Line Therapy: Artesunate IV 2.4mg/kg at 0h, 12h, 24h, then daily
Switch to oral therapy after at least 24 hours of IV therapy, once patient improving and can tolerate oral medication: Artemether-Lumefantrine (Riamet®) 20mg/120mg, 4 tablets at 0h, 8h, 24h, 36h, 48h and 60h N.B. Please note the timing of Riamet® doses relates to time from time zero – see worked example below:
N.B. Contact Pharmacy Department prior to discharge to ensure continuity of supply as Riamet® is not readily available in the community.
OR Quinine Sulphate 600mg TDS PO to complete total of 7 days PLUS start Doxycycline 100mg BD PO for 7 days (substitute Clindamycin 450mg TDS PO for 7 days if pregnant). |
|
Comments |
|
Malaria is a medical emergency. Always discuss with ID team or clinical microbiologist.
Diagnostic tests:
Admit patient medically if P. falciparum suspected or confirmed. Start treatment after laboratory confirmation except in severe disease with strong clinical suspicion. Patients who have taken malaria chemoprophylaxis should not receive the same drug for treatment.
Please see HPSC Clinical Guidelines on the Management of Suspected Malaria for further information, available at www.hpsc.ie .
Always document travel history for the past 12 months – countries and locations visited, travel dates, prophylaxis taken, prior history of malaria and co-morbidities. Malaria prophylaxis is not 100% effective and having taken prophylaxis does not rule out the possibility of malaria infection. The incubation period may be from 8 days up to 1 year. |
|
Duration of Treatment |
|
Duration of each agent as listed in the dosing section. |
|
Indication |
|
Malaria – Uncomplicated (Patient able to tolerate / retain tablets) Malaria species not identified or P. falciparum : If “species not identified” is subsequently diagnosed as P. vivax or P. ovale , see relevant table regarding treatment with primaquine. |
|
Antimalarial Treatment |
|
Artemether-Lumefantrine (Riamet®) 20mg/120mg: 4 tablets at 0h, 8h, 24h, 36h, 48h and 60h N.B. Please note the timing of Riamet® doses relates to time from time zero – see worked example below:
N.B. Contact Pharmacy Department prior to discharge to ensure continuity of supply as Riamet® is not readily available in the community.
OR Quinine Sulphate 600mg TDS PO PLUS Doxycycline 100mg BD PO for 7 days (Doxycycyline contra-indicated if pregnant, substitute Clindamycin 450mg TDS PO for 7 days)
OR Proguanil-Atovaquone (Malarone®) 100mg/250mg: 4 tablets daily PO for 3 days |
|
Comments |
|
Malaria is a medical emergency. Always discuss with ID team or clinical microbiologist.
Diagnostic tests:
Admit patient medically if P. falciparum suspected or confirmed. Start treatment after laboratory confirmation except in severe disease with strong clinical suspicion. Patients who have taken malaria chemoprophylaxis should not receive the same drug for treatment.
Please see HPSC Clinical Guidelines on the Management of Suspected Malaria for further information, available at www.hpsc.ie .
Always document travel history for the past 12 months – countries and locations visited, travel dates, prophylaxis taken, prior history of malaria and co-morbidities. Malaria prophylaxis is not 100% effective and having taken prophylaxis does not rule out the possibility of malaria infection. The incubation period may be from 8 days up to 1 year. |
|
Duration of Treatment |
|
Duration of each agent as listed in the dosing section. |
|
Indication |
|
Malaria – Non-falciparum P. vivax, P. ovale, P. malariae |
|
Antimalarial Treatment |
|
Treatment of malaria caused by P. vivax, P. ovale, P. malariae - chloroquine-SENSITIVE strains : Chloroquine 620mg at 0h, then 310mg at 6hr, 24h and 48h N.B . Chloroquine base 620mg = Chloroquine phosphate 1,000mg = 4 tablets of Avloclor®
PREVENTION OF RELAPSE i f malaria caused by P. vivax or P. ovale : Chloroquine should be FOLLOWED BY primaquine to eradicate parasites in the liver and thus prevent relapse: Primaquine 15mg PO daily (30mg PO daily if returned from Indonesia or Oceania) for 14 days N.B. Essential to screen for G6PD deficiency prior to commencing primaquine – primaquine can be started in the follow-up OPD appointment once result of G6PD deficiency screen available. N.B. Primaquine contra-indicated in pregnancy – discuss with ID Consultant if patient pregnant.
Treatment of malaria caused by P. vivax RESISTANT to chloroquine : Artemether-Lumefantrine (Riamet®) 20mg/120mg: 4 tablets at 0h, 8h, 24h, 36h, 48h and 60h N.B. Please note the timing of Riamet® doses relates to time from time zero – see worked example below:
N.B. Contact Pharmacy Department prior to discharge to ensure continuity of supply as Riamet® is not readily available in the community. FOLLOWED BY Primaquine a s above to prevent relapse
OR Quinine Sulphate 600mg TDS PO for 7 days PLUS Doxycycline 100mg BD PO for 7 days (substitute Clindamycin 450mg TDS PO if pregnant) FOLLOWED BY Primaquine as above to prevent relapse
OR Proguanil-Atovaquone (Malarone®) 100mg/250mg: 4 tablets daily PO for 3 days FOLLOWED BY Primaquine as above to prevent relapse
OR Mefloquine 10mg/kg PO at 0h and 8h (max 1.5g in 24hrs) FOLLOWED BY Primaquine as above to prevent relapse. |
|
Comments |
|
Malaria is a medical emergency. Always discuss with ID team or clinical microbiologist.
Diagnostic tests:
Patients who have taken malaria chemoprophylaxis should not receive the same drug for treatment.
Please see HPSC Clinical Guidelines on the Management of Suspected Malaria for further information, available at www.hpsc.ie .
Always document travel history for the past 12 months – countries and locations visited, travel dates, prophylaxis taken, prior history of malaria and co-morbidities. Malaria prophylaxis is not 100% effective and having taken prophylaxis does not rule out the possibility of malaria infection. The incubation period may be from 8 days up to 1 year. |
|
Duration of Treatment |
|
Duration of each agent as listed in the dosing section. |
Neutropenic Sepsis
|
Indication |
|
Neutropenic Sepsis: Initial Empiric Treatment |
|
First Line Antimicrobials |
|
Piperacillin/Tazobactam 4.5g QDS IV AND Gentamicin 5mg/kg daily IV (avoid gentamicin if myeloma - substitute ciprofloxacin 400mg BD IV instead) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. +/- If indwelling central vascular access device, MRSA or other gram positive infection suspected: Vancomycin 25mg/kg loading dose stat (max 3g), followed by15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. ADD Clarithromycin 500mg BD PO ( or Clarithromycin 500mg BD IV only where oral route is not feasible - excellent oral bioavailability) if evidence of community-acquired pneumonia. N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval (No need to add clarithromycin if patient on ciprofloxacin). If patient deteriorates clinically, contact Clinical Microbiologist for advice. |
|
Penicillin Hypersensitivity |
|
Ciprofloxacin 400mg BD IV N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. AND Gentamicin 5mg/kg daily IV (avoid gentamicin if myeloma) N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. If patient deteriorates clinically, contact Clinical Microbiologist for advice. |
|
Comments |
|
Neutropenic sepsis is a medical emergency. Contact Clinical Microbiologist, Oncologist and/or Haematologist for advice as required. N.B. Patients with neutropenic sepsis require daily clinical review. N.B. Review need for gentamicin daily. Avoid durations in excess of 5 days. Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. See also Policy for the Care and Management of Chemotherapy-Induced Neutropenia and Febrile Neutropenia in Adult Patients on network drive or in ward folder. |
|
Indication |
|
Neutropenic Sepsis: Persistent Fever on Empiric Antimicrobials |
|
First Line Antimicrobials |
|
If evidence of clinical deterioration:
If patient stable and culture negative:
If pathogen identified:
|
|
Duration of Treatment |
|
Continue treatment until patient is afebrile for at least 48 hours and/or neutrophil count has recovered. If patient remains neutropenic but is apyrexial for 48 hours and there is no clinical focus of infection, consider stopping antibiotics and observing patients in hospital for at least 48 hours. Restart antibiotics if patient becomes pyrexial again. |
Respiratory Tract Infections
|
Indication |
|
Aspiration Pneumonia – Community-Acquired |
|
First Line Antimicrobials |
|
Co-amoxiclav 1.2g TDS IV Empiric IV to PO switch: Co-amoxiclav 625mg TDS PO |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (or 500mg TDS IV only where oral route is not feasible - excellent oral bioavailability) Empiric IV to PO switch: Cefaclor LA 750mg BD PO AND Metronidazole 400mg TDS PO |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Levofloxacin 500mg BD PO or IV (excellent oral bioavailability) N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. Note – Levofloxacin has activity against oral anaerobes, therefore no need for additional metronidazole. |
|
Comments |
|
Microbiological Investigations:
Consider SALT referral. ALWAYS REVIEW empiric antimicrobial therapy in conjunction with C&S after 48 hours. N.B. Review patient for potential IV to PO switch after 48 hours. |
|
Duration of Treatment |
|
5 to 7 days |
|
Indication |
|
Aspiration Pneumonia – Hospital-Acquired
|
|
First Line Antimicrobials |
|
Piperacillin/Tazobactam 4.5g TDS IV If history of MRSA colonisation, ADD Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Penicillin Hypersensitivity |
|
Aztreonam 1g TDS IV AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline AND Metronidazole 400mg TDS PO (or 500mg TDS IV only where oral route is not feasible - excellent oral bioavailability) |
|
Comments |
|
Microbiological Investigations:
Consider SALT referral. ALWAYS REVIEW empiric antimicrobial therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
5 to 7 days |
|
Indication |
|
Community-Acquired Pneumonia (CAP): MILD - CURB-65 Score 0 to 1 N.B. Community-Acquired Pneumonia includes patients admitted from LTC facilities . |
|
First Line Antimicrobials |
|
Amoxicillin 1g TDS PO |
|
Penicillin Hypersensitivity |
|
Clarithromycin 500mg BD PO N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval OR Doxycycline 200mg once daily PO |
|
Comments |
|
N.B. Choice of antibiotic(s) should be guided by the CURB-65 score and clinical judgement of the severity of pneumonia. If the patient has respiratory sepsis, treat as severe CAP. CURB-65 score - 1 point for each criterion present:
|
|
Duration of Treatment |
|
5 days |
|
Indication |
|
Community-Acquired Pneumonia (CAP): MODERATE - CURB-65 Score 2 N.B. Community-Acquired Pneumonia includes patients admitted from LTC facilities . |
|
First Line Antimicrobials |
|
Amoxicillin 1g TDS PO (or 1g TDS IV only where oral route is not feasible - excellent oral bioavailability) AND Clarithromycin 500mg BD PO (or Clarithromycin 500mg BD IV only where oral route is not feasible - excellent oral bioavailability) N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval |
|
Penicillin Hypersensitivity |
|
Doxycycline 200mg once daily PO |
|
Comments |
|
N.B. Choice of antibiotic(s) should be guided by the CURB-65 score and clinical judgement of the severity of pneumonia. If the patient has respiratory sepsis, treat as severe CAP. CURB-65 score - 1 point for each criterion present:
Microbiological Investigations:
ALWAYS REVIEW empiric antimicrobial therapy in conjunction with C&S after 48 hours. N.B. Review patient for potential IV to PO switch after 48 hours. |
|
Duration of Treatment |
|
5 to 7 days |
|
Indication |
|
Community-Acquired Pneumonia (CAP): SEVERE - CURB-65 Score 3 to 5 N.B. Community-Acquired Pneumonia includes patients admitted from LTC facilities . |
|
First Line Antimicrobials |
|
Co-amoxiclav 1.2g TDS IV AND Clarithromycin 500mg BD PO (or Clarithromycin 500mg BD IV only where oral route is not feasible - excellent oral bioavailability) N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval Empiric IV to PO switch: Co-amoxiclav 625mg TDS PO AND Clarithromycin LA 1g once daily PO |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Clarithromycin 500mg BD PO (or Clarithromycin 500mg BD IV only where oral route is not feasible - excellent oral bioavailability) N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval Empiric IV to PO switch: Cefaclor LA 750mg BD PO AND Clarithromycin 500mg BD PO |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Levofloxacin 500mg BD PO or IV (excellent oral bioavailability) N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. |
|
Comments |
|
N.B. Empiric choice should be guided by the CURB-65 score and clinical judgement of the severity of pneumonia. If respiratory sepsis, treat as severe CAP. CURB-65 score - 1 point for each criterion present:
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. NB. Review patient for potential IV to PO switch after 48 hours. |
|
Duration of Treatment |
|
7 days |
|
Indication |
|
COVID-19 (SARS-CoV-2): Patients not requiring oxygen Paxlovid ® (nirmatrelvir/ritonavir) is not recommended for routine use in treatment of COVID-19. Treatment may be considered for selected seriously immunocompromised COVID-19 patients for the licensed indication. Licensed indication: For the treatment of COVID-19 in adults patients (over 18 years) who:
Reference: HSE Clinical Guidance on Paxlovid ® (nirmatrelvir/ritonavir) for use in the Treatment of COVID-19, Jan 2025 |
|
First Line Antimicrobials |
|
Paxlovid ® : Ritonavir 100mg PO BD + Nirmatrelvir 300mg PO BD Duration of treatment: 5 days Please note the Paxlovid ® packaging contains 1 x Ritonavir 100mg and 2 x Nirmatrelvir 150mg tablets for each dose. See picture below:
N.B. Dose adjustment required in renal impairment. See table below :
N.B. Paxlovid ® interacts with certain medications metabolised via CYP3A4 - refer to https://www.covid19-druginteractions.org/checker for full list. Contact the clinical pharmacy team and the Clinical Microbiology / Infectious Diseases / Respiratory team for assistance when considering a patient for Paxlovid ® Refer to the latest national guidelines, available at https://www.hse.ie/eng/about/who/acute-hospitals-division/drugs-management-programme/covid-19/ |
|
Comments |
|
Microbiological Investigations for suspected COVID-19:
Infection Prevention and Control:
|
|
Indication |
|
Severe COVID-19 (SARS-CoV-2): Patients with new oxygen requirement |
|
First Line Antimicrobials |
|
Contact the Clinical Microbiology / Infectious Diseases / Respiratory teams for advice on antiviral therapies (e.g. Remdesivir). Give Dexamethasone 6mg PO once daily for 7 - 10 days. Refer to the latest national guidelines, available at https://www.hse.ie/eng/about/who/acute-hospitals-division/drugs-management-programme/covid-19/ |
|
Comments |
|
Microbiological Investigations for suspected COVID-19:
Infection Prevention and Control:
|
|
Indication |
|
Hospital-Acquired Pneumonia
|
|
First Line Antimicrobials |
|
Piperacillin/Tazobactam 4.5g TDS IV If history of MRSA colonisation, ADD Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Penicillin Hypersensitivity |
|
Aztreonam 1g TDS IV AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
5 to 7 days |
|
Indication |
|
Infective Exacerbation of Asthma (IE Asthma) Antimicrobials are not always indicated. Many exacerbations are due to viral infection or environmental pollutants. |
|
First Line Antimicrobials |
|
Suspected IE Asthma without new CXR infiltrate: Amoxicillin 500mg TDS PO OR Clarithromycin 500mg BD PO N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval OR Doxycycline 200mg once daily PO If new infiltrate on CXR: Management the same as for severe CAP. |
|
Penicillin Hypersensitivity |
|
Suspected IE Asthma without new CXR infiltrate: Clarithromycin 500mg BD PO N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval OR Doxycycline 200mg once daily PO If new infiltrate on CXR: Management the same as for severe CAP. |
|
Comments |
|
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
5 to 7 days |
|
Indication |
|
Infective Exacerbation of Bronchiectasis (non-cystic fibrosis patients) |
|
First Line Antimicrobials |
|
Co-amoxiclav 625mg TDS PO or 1.2g TDS IV N.B . Consider risk of Pseudomonas aeruginosa if recent antimicrobial therapy or recent hospitalisation or history of cultures positive for Pseudomonas aeruginosa . |
|
Penicillin Hypersensitivity |
|
Clarithromycin 500mg BD PO N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval OR Doxycycline 200mg once daily PO N.B . Consider risk of Pseudomonas aeruginosa if recent antimicrobial therapy or recent hospitalisation or history of cultures positive for Pseudomonas aeruginosa . |
|
Comments |
|
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. Prior to discharge, refer to national immunisation guidelines for influenza and pneumococcal vaccinations, available at www.immunisation.ie . |
|
Duration of Treatment |
|
7 to 14 days |
|
Indication |
|
Infective Exacerbation of COPD (IE COPD) Antimicrobials are not always indicated. Many exacerbations are due to viral infection or environmental pollutants. Antimicrobials are indicated if the patient has at least two of the following:
OR Severe exacerbation requiring non-invasive or invasive mechanical ventilation. |
|
First Line Antimicrobials |
|
IE COPD without new CXR infiltrate: Amoxicillin 500mg TDS PO - if previous amoxicillin exposure within 3/12, use co-amoxiclav 625mg TDS PO If new infiltrate on CXR: Management the same as for severe CAP. |
|
Penicillin Hypersensitivity |
|
IE COPD without new CXR infiltrate: Clarithromycin 500mg BD PO N.B. Consider potential for drug interactions, e.g. statins, prolongation of QT interval OR Doxycycline 200mg once daily PO If new infiltrate on CXR: Management the same as for severe CAP. |
|
Comments |
|
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
5 to 7 days |
|
Indication |
|
Influenza (Flu) Treatment Clinical features: Sudden onset of fever ( > 38°C) or recent fever, cough, sore throat, limb or joint pain (two of these four symptoms are highly suggestive of influenza). Other symptoms can include rhinorrhoea, malaise, headache, vomiting or diarrhoea. |
|
First Line Antimicrobials |
|
Oseltamivir 75mg BD PO Treatment should be started as soon as possible, ideally within 48 hours of onset. ALWAYS refer to the latest national influenza management algorithm which is update regularly and available at http://www.hpsc.ie/a-z/respiratory/influenza/seasonalinfluenza/guidance/ |
|
Comments |
|
Previous influenza vaccination does not exclude influenza infection. Influenza typically circulates in the Northern Hemisphere between October and May. Latest information regarding national influenza activity is available at: http://www.hpsc.ie/a-z/respiratory/influenza/seasonalinfluenza/surveillance/ Microbiological Investigations:
Refer to OLOL IPCT Guidelines regarding need for infection control precautions. Public health notification is required for cases of influenza. Prior to discharge, refer to national immunisation guidelines for influenza and pneumococcal vaccinations, available at www.immunisation.ie . Consider prophylaxis for exposed contacts (see " influenza prophylaxis " section of guidelines app). |
|
Duration of Treatment |
|
5 days |
|
Indication |
|
Pleural Effusion with Pulmonary Infection / Suspected Empyema |
|
First Line Antimicrobials |
|
Co-amoxiclav 1.2g TDS IV |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (or 500mg TDS IV only where oral route not feasbile - excellent oral bioavailability) |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Contact clinical microbiologist for advice. |
|
Comments |
|
Microbiological Investigations:
Seek respiratory opinion regarding need for intercostal tube drainage. ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
Prolonged antimicrobial therapy may be required and duration depends upon successful drainage. |
|
Indication |
|
Pneumocytis jirovecii Pneumonia (PJP) |
|
First Line Antimicrobials |
|
N.B. ALWAYS contact clinical microbiologist / ID or respiratory teams for advice if PJP suspected.
Co-trimoxazole PO (excellent oral bioavailability) or IV only where oral route is not feasible Total daily dose of 120mg/kg given in 2 to 4 divided doses.
Example : Patient 85kg, normal renal function 120mg/kg x 85kg = 10,200mg total dose per day Each tablet/vial contains co-trimoxazole 480mg. 10,200mg divided by 480mg = 21.25 tablets/vials per day. Suggest round to 21 tablets/vials per day = 7 x 480mg TDS = 3,360mg TDS. |
|
Comments |
|
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
14 to 21 days |
|
Indication |
|
Respiratory Tuberculosis (TB) |
|
First Line Antimicrobials |
|
N.B. See BNF for further information on TB treatment. N.B. Check for drug interactions with rifampicin. Initial Phase (2 months duration):
Continuation Phase (4 months duration):
|
|
Comments |
|
Always contact Respiratory Consultant for advice. Microbiological Investigations:
Additional Investigations:
Contact Pharmacy prior to discharge to ensure continuity of supply on discharge. |
|
Duration of Treatment |
|
6 months minimum – ultimate duration depends on clinical and microbiological response and final mycobacterial culture and susceptibility results. |
Skin and Soft Tissue Infections
|
Indication |
|
Bursitis |
|
First Line Antimicrobials |
|
Management as for cellulitis – see relevant sections. If joint involvement, manage as for septic arthritis – see relevant sections. |
|
Indication |
|
Cellulitis – Mild to Moderate |
|
First Line Antimicrobials |
|
Flucloxacillin 1g QDS PO If history of MRSA colonisation: Discuss oral option with Microbiologist. |
|
Penicillin Allergy Alternatives |
|
Doxycycline 200mg once daily PO |
|
Comments |
|
Microbiological Investigations:
|
|
Duration of Treatment |
|
5 to 7 days. Cellulitis in the setting of lymphoedema may require up to 14 days. |
|
Indication |
|
Cellulitis – Severe |
|
First Line Antimicrobials |
|
Flucloxacillin 2g QDS IV Empiric IV to PO switch: Flucloxacillin 1g QDS PO If history of MRSA colonisation, SUBSTITUTE Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. IV to PO switch: Contact Clinical Microbiology for advice. |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-AZ-olin 2g TDS IV Empiric IV to PO switch: Cef-AL-exin 1g QDS PO If history of MRSA colonisation, SUBSTITUTE Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. IV to PO switch: Contact Clinical Microbiology for advice. |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Clindamycin 450mg QDS PO or 600mg QDS IV (excellent oral bioavailability) If history of MRSA colonisation, SUBSTITUTE Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. IV to PO switch: Contact Clinical Microbiology for advice. |
|
Comments |
|
N.B. If clinical suspicion of septicaemia, necrotising fasciitis or toxic shock syndrome, arrange urgent surgical review and contact the Clinical Microbiologist or ID Consultant for advice. Microbiological Investigations:
ALWAYS REVIEW empiric antimicrobial therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
7 to 14 days total including IV to PO switch |
|
Indication |
|
Diabetic Foot Ulcer: Infected SUPERFICIAL Ulcer |
|
First Line Antimicrobials |
|
Flucloxacillin 1g QDS PO |
|
Penicillin Allergy Alternatives |
|
Doxycycline 200mg once daily PO |
|
Comments |
|
Microbiological Investigations:
|
|
Duration of Treatment |
|
7 days |
|
Indication |
|
Diabetic Foot Ulcers: Infected DEEP Ulcer WITHOUT indications for hospitalisation Consider hospitalisation for management in the following situations:
|
|
First Line Antimicrobials |
|
Flucloxacillin 1g QDS PO AND Metronidazole 400mg TDS PO |
|
Penicillin Allergy Alternatives |
|
Doxycycline 200mg once daily PO AND Metronidazole 400mg TDS PO |
|
Comments |
|
Contact Podiatrist as soon as possible to facilitate sharp debridement, tissue sampling and appropriate offloading. Microbiological Investigations:
NB . Antimicrobials cannot penetrate into necrotic tissue. Contact Orthopaedic Team for review if osteomyelitis is suspected. |
|
Duration of Treatment |
|
10 to 14 days - ultimate duration depends on clinical presentation, extent of tissue involvement, adequate debridement, vascular supply and response to therapeutic interventions. |
|
Indication |
|
Diabetic Foot Ulcers: Infected DEEP Ulcer WITH indications for hospitalisation Consider involvement of the endocrinology team to facilitate rapid hospital discharge if the patient does not meet the criteria for in-patient management below. Consider hospitalisation for management in the following situations:
|
|
First Line Antimicrobials |
|
Co-amoxiclav 1.2g TDS IV SUBSTITUTE Piperacillin-tazobactam 4.5g TDS IV if:
If known history of MRSA colonisation, ADD Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV where oral route is not feasible ADD Gentamicin 5mg/kg daily IV if:
N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. If known history of MRSA colonisation, ADD Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral roue is not feasible |
|
Comments |
|
Contact Podiatrist as soon as possible to facilitate sharp debridement, tissue sampling and appropriate offloading. Microbiological Investigations:
N.B . Antimicrobials cannot penetrate into necrotic tissue. Contact Orthopaedic Team for review if osteomyelitis is suspected. |
|
Duration of Treatment |
|
10 to 14 days - ultimate duration depends on clinical presentation, extent of tissue involvement, adequate debridement, vascular supply and response to therapeutic interventions. |
|
Indication |
|
Diabetic Foot Osteomyelitis |
|
First Line Antimicrobials |
|
Co-amoxiclav 1.2g TDS IV SUBSTITUTE Pip/tazobactam 4.5g TDS IV if:
If known history of MRSA colonisation, ADD Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible ADD Gentamicin 5mg/kg daily IV if:
N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. If known history of MRSA colonisation, ADD Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible |
|
Comments |
|
Contact Podiatrist as soon as possible to facilitate sharp debridement, tissue sampling and appropriate offloading. Microbiological Investigations:
N.B . Antimicrobials cannot penetrate into necrotic tissue. Contact Orthopaedic Team for review if osteomyelitis suspected. |
|
Duration of Treatment |
|
Ultimate duration depends on clinical presentation, extent of tissue involvement, adequate debridement and vascular supply. |
|
Indication |
|
Herpes Zoster Infection (Shingles) |
|
First Line Antimicrobials |
|
Treatment is indicated for the following patient groups:
Valaciclovir 1g TDS PO |
|
Comments |
|
Always contact Clinical Microbiologist to discuss if herpes zoster meningitis or encephalitis is suspected. If an immunocompromised patient requires hospitalisation or if patient has severe neurological complications, discuss with neurology and Clinical Microbiologist. Microbiological Investigations:
|
|
Duration of Treatment |
|
7 days (in immunocompromised patient, continue for 2 days after crusting of lesions) |
|
Indication |
|
Human or Animal Bites |
|
First Line Antimicrobials |
|
Co-amoxiclav 625mg TDS PO |
|
Penicillin Allergy Alternatives |
|
Doxycycline 200mg daily PO AND Metronidazole 400mg TDS PO |
|
Comments |
|
Assess tetanus and rabies risk. Assess risk of blood borne viruses. |
|
Duration of Treatment |
|
5 days |
|
Indication |
|
Necrotising Skin and Soft Tissue Infections (Necrotising Fasciitis & Fournier's Gangrene) |
|
First Line Antimicrobials |
|
Piperacillin/Tazobactam 4.5g QDS IV AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. AND Clindamycin 1.2g QDS IV |
|
Penicillin Allergy Alternatives |
|
Ciprofloxacin 400mg BD IV N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 500mg TDS IV AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. AND Clindamycin 1.2g QDS IV |
|
Comments |
|
URGENT surgical review required. ALWAYS contact Consultant Microbiologist or ID Consultant for advice. Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
Ultimate duration depends on causative pathogen, clinical response, successful source control, blood culture results and absence of other deep foci of infection. |
|
Indication |
|
Penetrating trauma (e.g. stab wound or gunshot wound) |
|
First Line Antimicrobials |
|
Co-amoxiclav 1.2g TDS IV |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral roue is not feasible |
|
Comments |
|
Assess tetanus risk |
|
Duration of Treatment |
|
5 days |
|
Indication |
|
Surgical Site Infections – Clean Surgery |
|
First Line Antimicrobials |
|
Management the same as for cellulitis. |
|
Comments |
|
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
7 days including IV to PO switch |
|
Indication |
|
Surgical Site Infections – Clean-Contaminated or Dirty Surgery |
|
First Line Antimicrobials |
|
Piperacillin/Tazobactam 4.5g TDS IV |
|
Penicillin Allergy Alternatives |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Metronidazole 400mg TDS PO (excellent oral bioavailability) or 500mg TDS IV only where oral route is not feasible AND Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
7 days |
|
Indication |
|
Toxic Shock Syndrome |
|
First Line Antimicrobials |
|
ALWAYS contact Consultant Microbiologist or ID Consultant for advice. URGENT surgical review required if skin/ soft tissue source. |
|
Comments |
|
Microbiological Investigations:
|
|
Duration of Treatment |
|
Ultimate duration depends on causative pathogen, clinical response, successful source control, blood culture results and absence of other deep foci of infection. |
Urinary Tract Infections
|
Indication |
|
Asymptomatic Bacteriuria |
|
Comments |
|
Asymptomatic bacteriuria and positive urine dipsticks with leucocytes and / or nitrites and / or positive urine microbiology culture results are common in older patients and do not indicate UTI in the absence of clinical findings. Asymptomatic bacteriuria should ONLY be treated in pregnant women, children with abnormal urinary anatomy or patients undergoing urologic procedures. Treatment is not indicated in other groups. Inappropriate antimicrobial treatment can lead to the emergence of multi-drug resistant organisms and C. difficile infection. |
|
Indication |
|
Catheter-Associated Bacteriuria |
|
Comments |
|
|
Indication |
|
Cystitis / Lower UTI
|
|
First Line Antimicrobials |
|
If CrCl > 45 ml/min: Nitrofurantoin 50mg QDS PO If CrCl < 45 ml/min: Cef-AL-exin 500mg TDS PO If no oral alternative, nitrofurantoin may be used with caution when CrCl 30 to 45 ml/min, avoid if CrCl < 30 ml/min |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
If CrCl > 45 ml/min: Nitrofurantoin 50mg QDS PO If CrCl < 45 ml/min: Cef-AL-exin 500mg TDS PO If no oral alternative, nitrofurantoin may be used with caution when CrCl 30 to 45 ml/min, avoid if CrCl < 30 ml/min |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
If CrCl > 45 ml/min: Nitrofurantoin 50mg QDS PO If CrCl < 45 ml/min: Ciprofloxacin 500mg BD PO N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. If no oral alternative, nitrofurantoin may be used with caution when CrCl 30 to 45 ml/min, avoid if CrCl < 30 ml/min |
|
Comments |
|
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
Female – 3 days Male – 7 days |
|
Indication |
|
Urosepsis / Upper UTI/ Pyelonephritis
|
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g TDS IV AND Gentamicin 5mg/kg daily IV N.B. REVIEW NEED FOR GENTAMICIN DAILY. N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline.
IV to PO switch: Based on C&S |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g TDS IV AND Gentamicin 5mg/kg daily IV N.B. REVIEW NEED FOR GENTAMICIN DAILY. N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline.
IV to PO switch: Based on C&S |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Ciprofloxacin 500mg BD PO (excellent oral bioavailability) or 400mg BD IV only where oral route is not feasible N.B. Risk of long-lasting and disabling adverse effects with quinolones, mainly involving muscles, tendons and bones and the nervous system. Consider potential to prolong the QT interval. Consider that seizure threshold may be lowered. AND Gentamicin 5mg/kg daily IV N.B. REVIEW NEED FOR GENTAMICIN DAILY. N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline.
IV to PO switch: Based on C&S |
|
Comments |
|
N.B. Review need for gentamicin daily. Avoid durations in excess of 5 days.
Microbiological Investigations:
ALWAYS REVIEW empiric therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
7 to 10 days for urosepsis, 10 to 14 days for pyelonephritis including IV to PO switch . Duration 7 days for pyelonephritis if ciprofloxacin given. Ultimate duration dictated by clinical response, blood culture results, presence of pyelonephritis and adequate source control (e.g. abscess drainage). |
|
Indication |
|
Post-TRUS Prostate Biopsy Urosepsis/ UTI |
|
First Line Antimicrobials |
|
Urosepsis: Piperacillin/Tazobactam 4.5g TDS IV AND Amikacin 15mg/kg daily IV N.B. REVIEW NEED FOR AMIKACIN DAILY. N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for CrCl calculator and amikacin guideline.
UTI without sepsis: If CrCl > 45 ml/min: Nitrofurantoin 100mg QDS PO If CrCl < 45 ml/min: Contact Clinical Microbiologist for advice. If no oral alternative, can use nitrofurantoin with caution when CrCl 30 to 45ml/min, avoid if CrCl < 30 ml/min.
IV to PO switch: Based on C&S |
|
Penicillin Hypersensitivity |
|
Urosepsis: Contact Clinical Microbiologist for advice. |
|
Comments |
|
N.B. Review need for amikacin daily. Avoid durations in excess of 5 days.
Microbiological Investigations:
ALWAYS REVIEW empiric antimicrobial therapy in conjunction with C&S after 48 hours. |
|
Duration of Treatment |
|
7 to 14 days - ultimate duration dictated by clinical response, blood culture results. |
Vascular Catheter Infections
|
Indication |
|
Peripheral Vascular Catheter (PVC) Infection |
|
First Line Antimicrobials |
|
Flucloxacillin 2g QDS IV
If history of MRSA colonisation, SUBSTITUTE Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
NON-immediate-onset and NON-severe Penicillin Hypersensitivity |
|
Cef-AZ-olin 2g TDS IV
If history of MRSA colonisation, SUBSTITUTE Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Clindamycin 450mg QDS PO or 600mg QDS IV (excellent oral bioavailability)
If history of MRSA colonisation, SUBSTITUTE Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
REMOVE THE INFECTED PVC IMMEDIATELY.
PVCs are a portal of entry for Staph. aureus . PVC infections can manifest as local phlebitis or bloodstream infections. The risk of PVC infection may be reduced by:
Microbiological Investigations:
|
|
Duration of Treatment |
|
If blood cultures positive for S. aureus :
If phlebitis with sterile blood cultures:
|
|
Indication |
|
Central Vascular Catheter (CVC) Infection |
|
First Line Antimicrobials |
|
Local CVC exit site infection in systemically well patient: Line removal and topical antiseptic care may be sufficient. If antimicrobials indicated, please contact Clinical Microbiologist to discuss.
Systemically unwell patient with suspected CVC infection (please note CVC exit site may appear normal): Vancomycin 25mg/kg loading dose (max 3g), followed by 15mg/kg BD IV N.B. Adjust dose if renal impairment, trough level monitoring required, click on link above for calculator and guideline. |
|
Comments |
|
CAN THE CVC BE REMOVED? Always discuss with senior clinician.
Microbiological Investigations:
|
|
Duration of Treatment |
|
Duration depends on blood culture results, pathogens isolated, clinical response and absence of deep-seated focus of infection (e.g. endocarditis) on further investigation. |
Voriconazole Prescribing Aid
Voriconazole Prescribing Aid
Please see BNF or SPC for full prescribing information.
Dose :
IV: Loading dose 6 mg/kg IV every 12 hours for 2 doses, then maintenance dose 4 mg/kg IV every 12 hours. 1-3
Oral: N.B. Oral voriconazole must be taken on an empty stomach for effect.
If patient is on NG feed, a 3 hour feedbreak is required for each dose: Stop NG feed 2 hours before dose, restart NG feed 1 hour after dose. 4
Adult (body-weight up to 40 kg): Initially 200 mg PO every 12 hours for 2 doses, then 100 mg PO every 12 hours, increased if necessary to 150 mg PO every 12 hours. 1-3
Adult (body-weight 40 kg and above): Initially 400 mg PO every 12 hours for 2 doses, then 200 mg PO every 12 hours, increased if necessary to 300 mg PO every 12 hours. 1-3
Dose in Renal Impairment :
No dose adjustment required in renal impairment or CRRT. 5 When CrCl < 50 mL/min, accumulation of the intravenous vehicle may occur. Monitor renal function if patient on IV treatment. 1,5
Use oral route unless risk/benefit justifies use of IV, e.g. check if patient absorbing PO/NG medicines.
Drug Interactions :
-
Voriconazole can increase QTc – monitor, particularly if patient on other medication which can increase QTc. 6
-
Simvastatin: Voriconazole increases exposure to simvastatin. Suggest HOLD (combination contra-indicated). 6
-
Atorvastatin: Voriconazole increases exposure to atorvastatin. Suggest HOLD (manufacturer advises to avoid or use a lower maximum dose and monitor for rhabdomyolysis). 6
-
Benzodiazepines: Voriconazole is likely to increase the concentration of benzodiazepines and lead to a prolonged sedative effect, dose reduction may be required. 6
-
Phenytoin decreases the exposure to voriconazole and voriconazole increases exposure to phenytoin. Manufacturer advises avoid or adjust voriconazole dose and monitor phenytoin level. 6
-
Multiple other interactions, see BNF or SPC.
Therapeutic Drug Monitoring :
-
Check trough level after 3 to 5 days. 7
-
Use serum bottle brown top tube
-
Repeat level in the second week to ensure it is in therapeutic range. 7
-
In practice, send level on Monday so that Laboratory Referrals can send out on Tuesday morning for processing on Wednesday. Result usually available by Friday evening.
Other Monitoring :
-
Monitor ALT and AST 1-3
-
Monitor for rash – risk of SJS or TEN with voriconazole. 2,3
-
Reports of prolonged visual adverse reactions, including blurred vision, optic neuritis and papilloedema. 2,3
-
Risk of phototoxicity. 1-3
References :
-
British National Formulary. Available from www.medicinescomplete.com , accessed 22/2/21.
-
Fresenius Kabi.Summary of Product Characteristics for Voriconazole 200mg powder for solution for infusion. 2020. Available from www.hpra.ie , accessed 22/2/21.
-
Pfizer. Summary of Product Characteristics for Vfend® 50mg and 200mg tablets. 2020. Available from www.hpra.ie , accessed 22/2/21.
-
The NEWT Guidelines. Available from www.newtguidelines.com , accessed 22/2/21.
-
The Renal Drug Database.Available from www.renaldrugdatabase.com , accessed 22/2/21.
-
Stockley’s Drug Interactions.Available from www.medicinescomplete.com , accessed 22/2/21.
-
ECIL 6 Meeting. Triazole Antifungal Therapeutic Drug Monitoring. Final slide set posted on ECIL website Dec 8 th , 2015. On file in OLOL Pharmacy Department.
Document prepared by: Carmel McKenna; Checked by: Catriona Campbell; OLOL Pharmacy Department Feb 2021.
References
- RCPI Hospital Antimicrobial Stewardship Working Group 2012. Start Smart then Focus Antibiotic Care Bundle. Reproduced with permission. Available from www.rcpi.ie .
- Beaumont Hospital Antimicrobial Guidelines 2020. Available from RCSI Hospitals Antimicrobial Guidelines Smartphone Application, accessed 31/03/2020. Obtained with permission from Clinical Microbiologist.
- NICE Guideline CG183. Drug allergy: diagnosis and management of drug allergy in adults, children and young people. 2014. Available from www.nice.org.uk .
- Irish Medication Safety Network. Briefing Document: Reducing preventable harm to patients with known drug allergies, 2012. Available from www.imsn.ie .
- BMJ Group and Pharamceutical Press. British National Formulary online. Available from www.medicinescomplete.com , accessed 12/03/18.
- McKenna C. Medicines Information Enquiry on cross-reactivity of penicillin and meropenem, Mar 2014. On file in OLOL Pharmacy.
- Frumin J, Gallagher JC. Allergic cross-sensitivity between penicillin, carbapenem, and monobactam antibiotics: what are the chances? Ann Pharmacother 2009;43:304-15.
- Cunha BA , Hamid NS, Krol V et al. Safety of meropenem in patients reporting penicillin allergy: lack of allergic cross reactions J Chemother. 2008;20(2):233-7.
- Romano A, Viola M, Gueant-Rodriquez RM et al. Brief communication: tolerability of meropenem in patients with IgE-mediated hypersensitivity to penicillins. Ann Intern Med 2007;146(4):266-9.
- Romano A, Gaeta F, Valluzzi RL et al. Absence of cross-reactivity to carbapenems in patients with delayed hypersensitivity to penicillins. Allergy 2013;68(12):1618-21.
- Sanford Guide to Antimicrobial Therapy, available from webedition.sanfordguides.com, accessed 12/03/18.
- John Hopkins ABX Guide, available from www.hopkinsguides.com , accessed 12/03/18.
- Truven Health Analytics Inc. Micromedex® Medication, Disease and Toxicology Management. Available from www.micromedexsolutions.com , accessed 9/5/16.
- SARI Hospital Antimicrobial Stewardship Working Group. Guidelines for Antimicrobial Stewardship in Hospitals in Ireland, 2009. Available from www.hpsc.ie .
- Health Products Regulatory Authority. Summary of Product Characteristics for each product available from www.hpra.ie , accessed 10/6/14.
- National Clinical Effectiveness Committee. Sepsis Management for Adults (including Maternity). National Clinical Guideline No. 26, Version 2, 2025. Available from www.hse.ie .
- Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2021. Crit Care Med 2021; 49(11):e1063-e1143.
- Irish Medication Safety Network. Safety Alert: Rhabdomyolysis with fusidic acid and statins, 2012. Available from www.imsn.ie .
- Osmon DR, Berbari EF, Berendt AR et al. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013;56(1):e1–25.
- Pfizer Limited. Summary of Product Characteristics for Diflucan® 50mg capsules (fluconazole), Nov 2017. Available from www.hpra.ie .
- Habib G, Lancellotti P, Antunes MJ, et al: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). 2015 ESC Guidelines for the management of infective endocarditis. Eur Heart J 2015;36:3075–3123.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications. A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132:1435-1486.
- Scientific Advisory Committee, HPSC. Guidelines for the Early Clinical and Public Health Management of Bacterial Meningitis, 2012, revised 2016. Available from www.hpsc.ie .
- Brouwer MC, McIntyre P, Prasad K, et al. Corticosteroids for acute bacterial meningitis. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No.: CD004405. DOI: 10.1002/14651858.CD004405.pub5.
- Clostridioides difficile infection - Irish National Guidelines, April 2023. Available from www.hse.ie.
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection – the Maastrict V / Florence Consensus Report. 5 th Edition. Gut 2016;0:1–25.
- Smith S, Boyle B, Brennan D, et al. The Irish Helicobacter pylori Working Group consensus for the diagnosis and treatment of H. pylori infection in adult patients in Ireland. European Journal of Gastroenterology and Hepatology 2017; 29(5):552 – 559.
- Low J. Dublin North East Genitourinary Medicine Guidelines, 2011. Available on OLOL T drive, OLH J drive, LCH ward folder.
- Street E, Joyce A, Wilson J. 2010 United Kingdom national guideline for the management of epididymo-orchitis. Clinical Effectiveness Group, British Association for Sexual Health and HIV. Available from www.bashh.org .
- NICE Guideline NG 110. Prostatitis (acute): antimicrobial prescribing. Oct 2018. Available from www.nice.org.uk .
- Grabe M, Bishop MC, Bjerklund-Johansen TE et al. Guidelines on the management of urinary and male genital tract infections 2008. European Association of Urology. Available from www.uroweb.org .
- Ross J, Cole M, Evans C, et al. United Kingdom National Guideline for the Management of Pelvic Inflammatory Disease (2019 interim update). Clinical Effectiveness Group, British Association for Sexual Health and HIV. Available from www.bashh.org .
- Solomkin JS, Mazuski JE, Bradley JS et al. Diagnosis and Management of Complicated Intra-abdominal Infection in Adults and Children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010;50:133-164.
- Mazuski JE, Tessier JM, May AK et al. The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection. Surgical Infections. Jan 2017.1-76.
- Villatoro E, Mulla M, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database of Systematic Reviews 2010. Available from www.cochranelibrary.com .
- UK Working Party on Acute Pancreatitis. UK guidelines for the management of acute pancreatitis. Gut 2005;54(Suppl III):iii1–iii9.
- Runyon BA. Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. American Association for the Study of Liver Diseases. Available from www.aasld.org .
- HPSC and Beaumont Hospital. Clinical Guidelines on the management of suspected malaria. 2017. Available from www.hpsc.ie .
- Royal College of Obstetricians and Gynaecologists. Green-Top Guideline No. 54B. The diagnosis and treatment of malaria in pregnancy, 2010. Available from www.rcog.org.uk .
- Lalloo DG, Shingadia D, Bell DJ, et al. UK Malaria Treatment Guidelines 2016. J Infect 2016;72:635-649.
- Freifeld AG, Bow EJ, Sepkowitz KA et al. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 2011;52(4):e56–e93.
- Averbuch D, Orasch C, Cordonnier C, et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4 th European Conference on Infections in Leukemia. Haematologica . 2013;98(12): 1826–1835.
- Lim WS, Baudouin SV, George RC et al, British Thoracic Society Standards of Care Committee. Guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64(Suppl III):s1-55.
- NICE Guideline CG191: Pneumonia in adults: diagnosis and management, 2019. Available from www.nice.org.uk .
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clinical Infectious Disease 2016;63(5):e61–111.
- NICE Guideline NG139. Pneumonia (hospital-acquired): antimicrobial prescribing. Sep 2019. Available from www.nice.org.uk
- Scottish Intercollegiate Guidelines Network. SIGN158: British guideline on the management of asthma. 2019. Available from www.sign.ac.uk .
- Global Strategy for the diagnosis, management and prevention of COPD, Global Initiative For Chronic Obstructive Lung Disease (GOLD) 2018. Available from www.goldcopd.org .
- NICE Guideline NG114: Chronic obstructive pulmonary disease (acute exacerbation): antimicrobial prescribing. 2018. Available from www.nice.org.uk .
- HSE and HPSC. Guidance on the use of antiviral agents for the treatment and prophylaxis of influenza, 2017-2018. Available from www.hpsc.ie .
- National TB Advisory Committee. Guidelines on the prevention and control of tuberculosis in Ireland, 2010. Available from www.hpsc.ie .
- Stevens DL, Bisno AL, Chambers HF et al. Practice Guidelines for the diagnosis and management of skin and soft-tissue infections: 2014 Update by the Infectious Diseases Society of America. IDSA Guidelines. Clin Infect Dis 2014;59(2):147-59.
- Lipsky BA, Berendt AR, Deery HG et al. 2012 Infectious Diseases Society of America Clinical Practice Guidelines for the diagnosis and treatment of Diabetic Foot Infections. Clin Infect Dis 2012;54:132-73.
- Gupta K, Hooton TM, Naber KG et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011;52(5):e103–e120.
- National Cancer Control Programme National Prostate Biopsy Infection Project Board. National policy on the prevention and management of infection post transrectal ultrasound (TRUS) guided prostate biopsy, 2014. Available from www.hse.ie .
- Pinewood Laboratories Limited. Flucillin® (Flucloxacillin) 500mg capsules, Summary of Product Characteristics. May 2015. Available from www.hpra.ie , accessed 9/5/16.
- Gill D, Bates M, Bonner C et al. Immunisation Guidelines for Ireland. Available from www.immunisation.ie , accessed 17/10/18.
- Davies JM, Lewis MP, Wimperis J et al. Review of guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen: Prepared on behalf of the British Committee for Standards in Haematology by a Working Party of the Haemato-Oncology Task Force. Br J Haematol 2011;155:308-317.
- Working Party of the British Committee for Standards in Haematology Clinical Haematology Task Force. Guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. Br Med J 1996;312:430.
- Occupational Health Department. Meningococcal Disease Prevention: For Employees of the Health Service Executive, Dublin North East (HSE DNE). 2016. On file on OLOL T Drive.
- Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal Hypertensive Bleeding in Cirrhosis: Risk Stratification, Diagnosis, and Management: 2016 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2017;65(1):310-335.
- RCPI/RCSI Working Group on Prevention of Surgical Site Infections. Preventing Surgical Site Infections: Key recommendations for practice, 2012. Available from www.rcpi.ie .
- Scottish Intercollegiate Guidelines Network. SIGN 104: Antibiotic prophylaxis in surgery. 2014. Available from www.sign.ac.uk .
- Bratzler DW, Dellinger ED, Olsen KM et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013;70:195-283.
- Wockhardt UK Limited. Co-amoxiclav 1000mg/200mg Summary of Product Characteristics, 2015. Available from www.hpra.ie , accessed 9/5/16.
- American Society for Gastrointestinal Endoscopy. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2008;67(6):791-6.
- Allison MC, Sandoe JAT, Tighe R et al. Antibiotic prophylaxis in gastrointestinal endoscopy. British Society of Gastroenterology. Gut 2009;58:869–880.
- Hoff WS, Bonadies JA, Cachecho R, et al. East Practice Management Guidelines Work Group: Update to Practice Management Guidelines for Prophylactic Antibiotic Use in Open Fractures. J Trauma 2011;70(3):751-754.
- A Strategy for the Control of Antimicrobial Resistance in Ireland (SARI). Guidelines for the prevention of catheter-associated urinary tract infection. 2011. Available from www.hpsc.ie .
- Wolf JS, Bennett CJ, Dmochowski RR et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. American Urological Association, 2014. Available from www.auanet.org .
- McKenna C. Medicines Information Enquiry on which weight to use for creatinine clearance, 2014. On file in OLOL Pharmacy.
- CRC Press, Taylor and Francis Group. The Renal Drug Database. Available from www.renaldrugdatabase.com , accessed 9/5/16.
- Nicolau DP, Freeman CD, Belliveau PP et al. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 1995;39(3):650-655.
- Rybak M, Lomaestro B, Rotschafer JC et al. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009;66:82-98.
- National Clinical Programme for OPAT. Available from www.opat.ie , accessed 21/8/14.
- HSE / HPSC. List of Notifiable Disease. Available from www.hpsc.ie/notifiablediseases , accessed 9/5/16.
- Mosby's Medical Dictionary, 8th edition. © 2009, Elsevier. Available from http://medical-dictionary.thefreedictionary.com , accessed 10/6/14.
- Brigg Turner R, Cumpston A, Sweet M et al. Prospective, Controlled Study of Acyclovir Pharmacokinetics in Obese Patients. Antimicrob Agents Chemother 2016;60(3):1830-1833.
- Cook T, Harper N [Editors]. Anaesthesia, Surgery and Life-Threatening Allergic Reactions. Report and Findings of the Royal College of Anaesthetists’ 6 th National Audit Project: Perioperative anaphylaxis. RCoA, HSRC and NAP; May 2018. Available from www.nationalauditprojects.org.uk .
- Fifer H, Saunders J, Soni S, et al [on behalf of Clinical Effectiveness Group, BASHH]. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae . International Journal of STD & AIDS 2020;31(1):4–15. Available from www.bashh.org .
- Saxon C, Edwards A, Rautemaa-Richardson R, et al [on behalf of Clinical Effectiveness Group, BASHH]. British Association for Sexual Health and HIV national guideline for the management of vulvovaginal candidiasis (2019). Available from www.bashh.org , accessed 23/06/2020.
- Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro BM. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020 May 19;77(11):835-864. doi: 10.1093/ajhp/zxaa036. PMID: 32191793.
- Knight M, Chiocchia V, Partlett C, Rivero-Arias O, Hua X, Hinshaw K, Tuffnell D, Linsell L, Juszczak E; ANODE collaborative group. Prophylactic antibiotics in the prevention of infection after operative vaginal delivery (ANODE): a multicentre randomised controlled trial. Lancet. 2019 Jun 15;393(10189):2395-2403. doi: 10.1016/S0140-6736(19)30773-1. Epub 2019 May 13. Erratum in: Lancet. 2019 Jun 15;393(10189):2394. PMID: 31097213; PMCID: PMC6584562.
- Krutova M, Norén T, Allerberger F, Coia J, Goorhuis A, van Rossen TM, Ooijevaar RE, Burns K, Scharvik Olesen BR, Tschudin-Sutter S, Wilcox MH, Vehreschild M, Fitzpatrick F, Kuijper EJ, The guideline committee of the European Study Group on Clostridioides difficile, European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults, Clinical Microbiology and Infection, https://doi.org/10.1016/