General Information
Contact Information
Our Lady of Lourdes Hospital and Louth County Hospital
Medical Microbiology and Infectious Diseases OLOL and LCH
|
Dr Marta Trzos-Grzybowska, Consultant Microbiologist |
Mobile via Switch |
|
Dr Binu Dinesh, Consultant Microbiologist |
Mobile via Switch |
|
Dr Roisin Connolly, Consultant Microbiologist |
Mobile via Switch |
|
Dr Justin Low, Infectious Diseases Consultant |
Mobile via Switch |
|
Dr Brendan O'Kelly, Infectious Diseases Consultant |
Mobile via Switch |
|
Microbiology Registrar |
Ext. 2104/ 2393 Bleep 736 |
OPAT Referrals
|
Contact ID Team directly |
Pharmacy Departments OLOL and LCH
|
Goretti Ngige / Carmel McKenna Antimicrobial Pharmacist, Our Lady of Lourdes Hospital, Drogheda |
Bleep 483 |
|
Pharmacy Department, Our Lady of Lourdes Hospital, Drogheda |
Extn. 4663 Extn. 4610 |
|
Pharmacy Department, Louth County Hospital, Dundalk |
Extn. 5441 |
Our Lady's Hospital Navan
Medical Microbiology OLH Navan
|
Consultant Microbiologist |
Mobile via switch |
Pharmacy Department OLH Navan
|
Anna Marzec Antimicrobial Pharmacist, Our Lady’s Hospital, Navan |
Bleep 712 |
|
Pharmacy Department, Our Lady’s Hospital, Navan |
Extn. 2553 |
Guideline Purpose and Scope
Guideline Purpose
The purpose of the guideline is to promote rational antimicrobial prescribing in the acute hospital setting with effective therapies tailored to clinically significant microbiology results for the minimum effective duration to avoid healthcare-associated infection and the development of antimicrobial resistance and promote the rapid and effective treatment with a minimum of adverse effects.
Guideline Scope
The guideline applies to medical, nursing and pharmacy staff in LH. It serves as a guide to antimicrobial prescribing, however it is not intended to meet the clinical needs of every individual patient. Adherence to this guideline will not ensure successful outcome in every case. It does not include all proper methods of care or exclude other acceptable methods of care. The final decision on antimicrobial prescribing must be made by the clinician responsible for the patient. The Consultant Microbiologists and Infectious Diseases (ID) Consultant may be contacted for further advice when required.
The guideline applies to non-pregnant adults only unless otherwise specified. Please consult the antimicrobial guidelines for obstetric patients in the " Obstetrics and Gynaecology " tile and antimicrobial guidelines for neonates and paediatrics in the " Neonatal and Paediatrics " tile. The guideline covers empiric treatment of common and serious infections. Prophylaxis of infection, mainly surgical prophylaxis, is also included. The guideline is not intended to be a comprehensive guide to the clinical or laboratory diagnosis of infection. Non-antibiotic treatments, infection control and clinical care pathways are outside the scope of the guideline. Some recommendations involve unlicensed medicines or an off-label use of a licensed medicine. This remains the responsibility of the prescriber.
The doses recommended in the guideline are standard adult doses and may need to be altered with regard to weight, renal function, hepatic function, drug contra-indications, drug interactions and adverse effects. The prescriber is responsible for making themselves aware of and taking this information into account. The " Obstetrics and Gynaecology " tile provides a list of useful reference sources for checking the safety of antimicrobials in pregnancy and lactation. The prescriber has final responsibility for the antimicrobials prescribed for pregnant and breast-feeding patients. Pharmacy may be contacted for further information if required.
Roles and Responsibilities
This document is a guideline only. The final decision on antimicrobial prescribing must be made by the prescriber treating the patient based on clinical information and the diagnostic and treatment options available.
Prescribing Principles
- See RCPI “ Start Smart then Focus ” Antibiotic Care bundle for a summary of prescribing principles.
- If the patient is pregnant or breast-feeding , the prescriber should check the stage of pregnancy and the safety of each antimicrobial for use in pregnant or breast-feeding patients.
- N.B . Always review empiric therapy after 48 hours in conjunction with culture and sensitivity (C&S) results.
- The ideal antibiotic is effective, safe and cost-effective, does not induce resistance or cause healthcare-associated infection.
- The development of Clostridium difficile infection is of particular concern and is associated with the inappropriate use of antimicrobial agents.
- Penicillin and related hypersensitivity must always be considered when prescribing antibiotics.
- High dose therapy for a shorter period is more effective than low dose therapy for a protracted course.
- Antibiotic review date / planned duration should be documented on the drug chart and in the medical notes.
- Antibiotics are useful for treating bacterial infection. However in situations where source control (i.e. drainage of an abscess or relief of an obstruction) is required, antibiotics are only useful as an adjunct to these measures.
- Intramuscular administration should be avoided except in specific circumstances, eg. sexually transmitted infections, transrectal prostate biopsy antibiotic prophylaxis.
- The use of topical antimicrobial agents should be discouraged as this encourages development of resistance.
- All serious adverse drug reactions (ADR) should be reported to the Health Products Regulatory Authority (HPRA), previously known as the Irish Medicines Board. The ADR should be reported electronically on www.hpra.ie . The reported ADR and any resulting correspondence should also be recorded in the patient’s medical notes.
- See relevant section on antibiotics that are subject to restricted use .
- This guideline should be made available to locum and agency prescribing staff as well as permanent staff.
- Any deviation in the use of antimicrobials from this guideline should be clearly documented in the patient’s medical record.
- If the Consultant Microbiologist or ID Consultant is contacted concerning patient management, this should be by a senior medical staff member when possible, i.e. Registrar or Consultant.
Start Smart then Focus Antibiotic Care Bundle
Start Smart then Focus Antibiotic Care Bundle
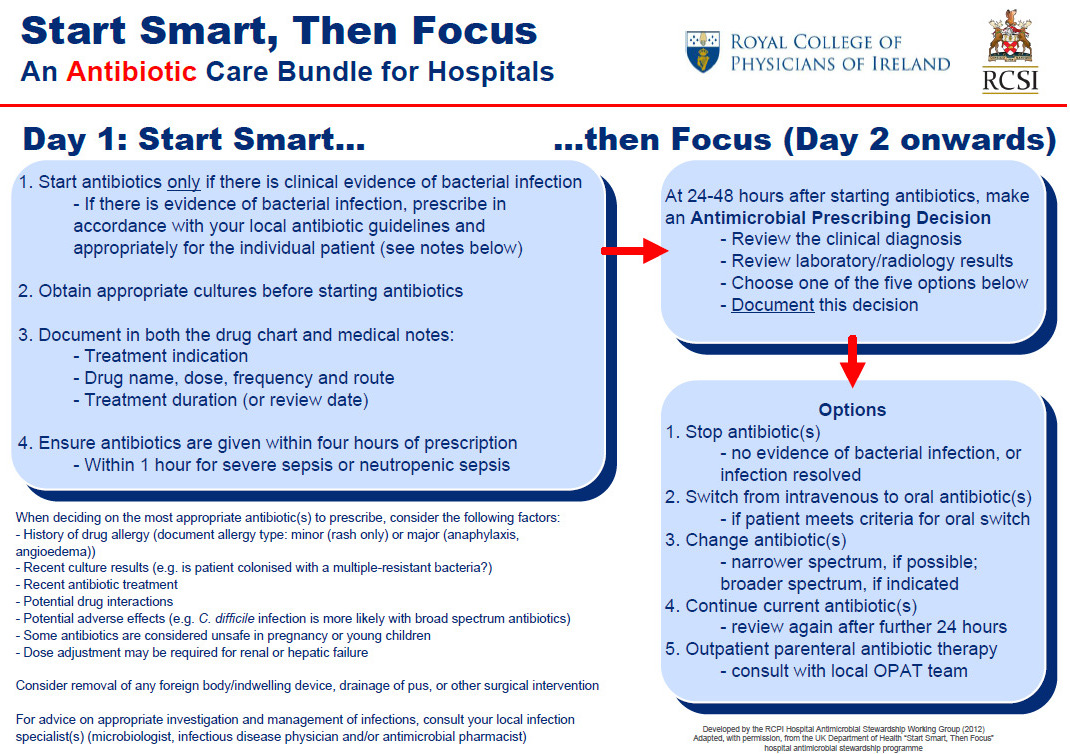
Reproduced with permission from RCPI Hospital Antimicrobial Stewardship Working Group
Notifiable Diseases
See HPSC Notifiable Diseases List for full list of notifiable diseases.
The medical or surgical team under which the patient is admitted must notify Public Health of all clinically suspected or proven cases of infectious diseases on the list using the HPSC Standard Notification Form .
Paper copies of the form may be found on the ward .
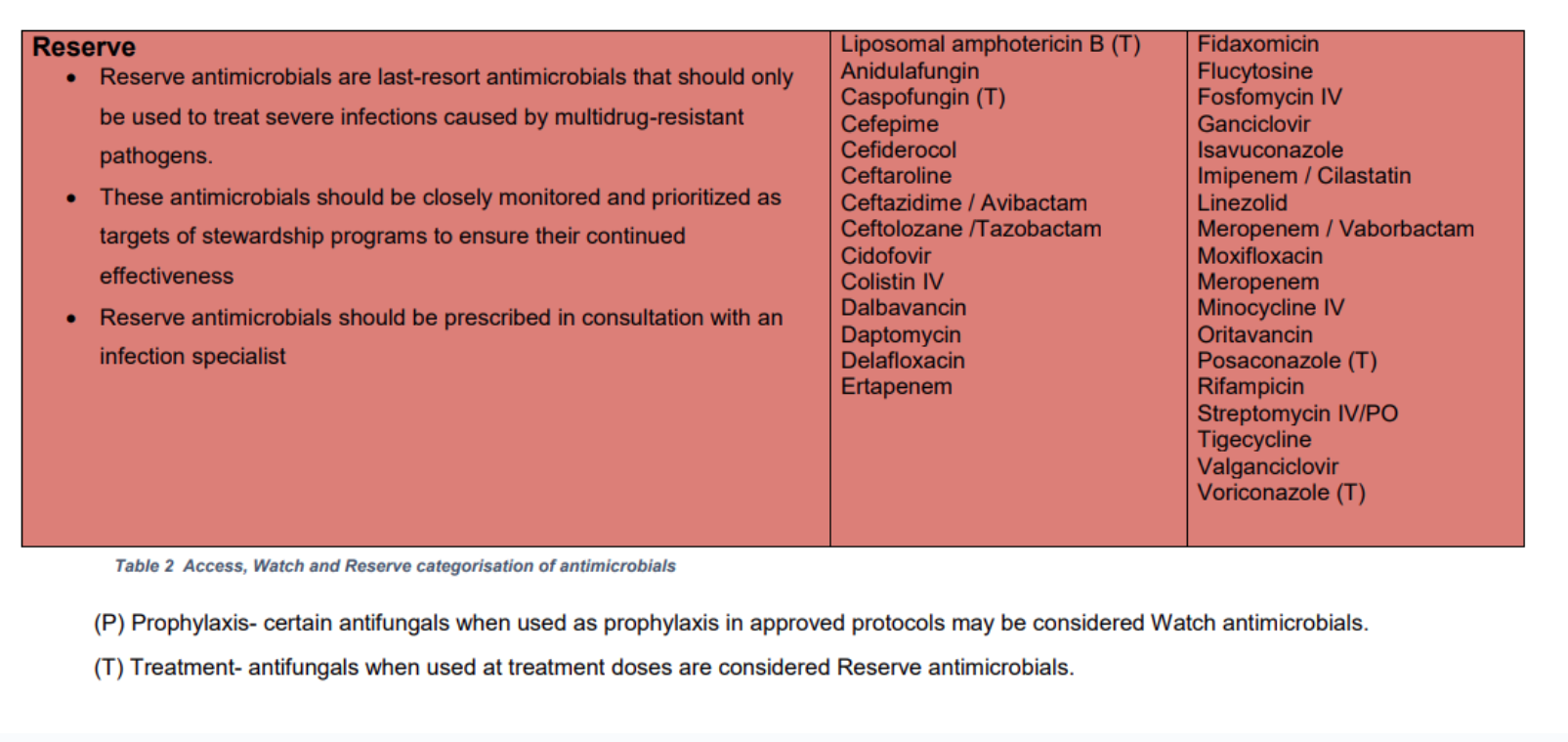
Reserve Antimicrobials
In Louth Hospitals (LH), reserve antimicrobials should be prescribed only in the following cases:
- When recommended by the LH antimicrobial guidelines
- When pre-authorised by the clinical microbiology or infectious diseases (ID) teams, i.e. advice should be sought BEFORE prescribing a reserve antimicrobial.
The LH Reserve Antimicrobials are listed in the table below (same as HSE AMRIC list).
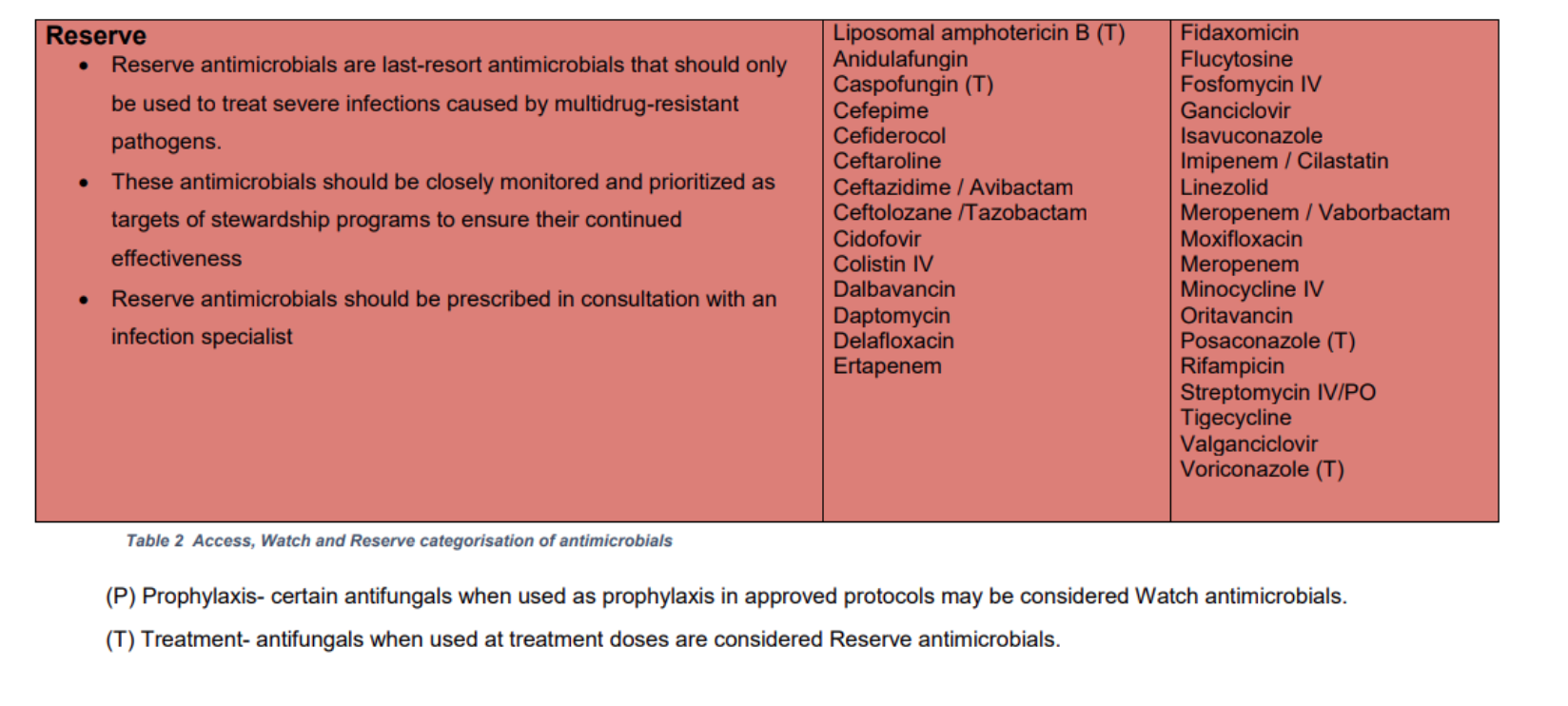
Access, Watch and Reserve 'AWaRe' Antimicrobials
The LH Reserve Antimicrobials Policy is based on the HSE AMRIC Reserve Antimicrobials National Policy, Dec 2024. The World Health Organisation (WHO) Access, Watch and Reserve ‘AWaRe’ categorisation of antimicrobials was adapted by AMRIC for the purpose of this policy.
Table 1. WHO Definition of Access, Watch and Reserve Antimicrobials.

Table 2. List of LH Access Antimicrobials (same as HSE AMRIC List).
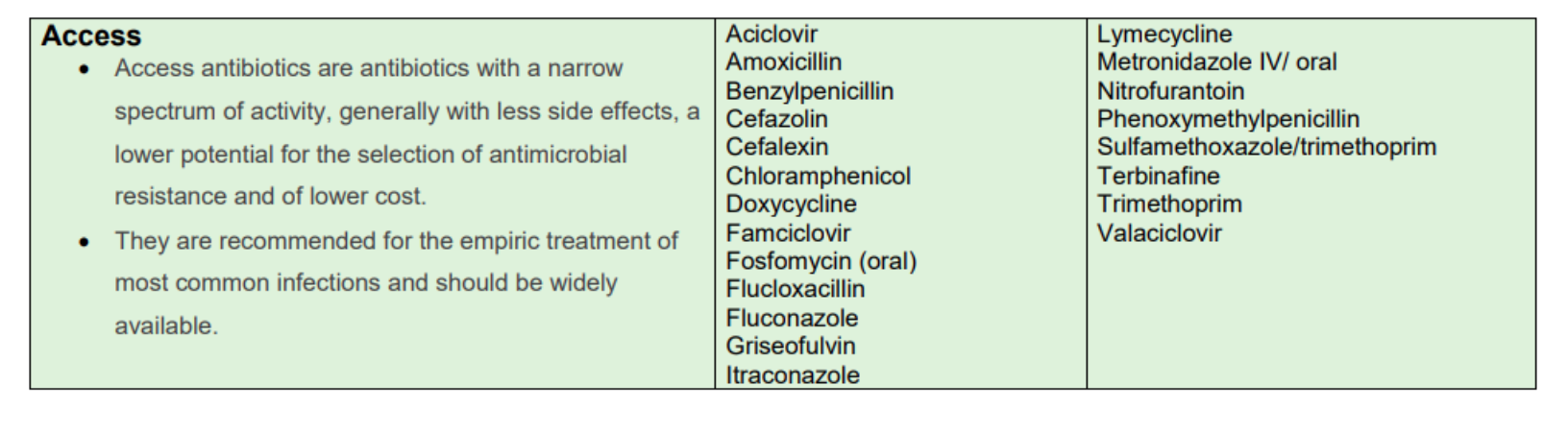
Table 3. List of LH Watch Antimicrobials (same as HSE AMRIC List).
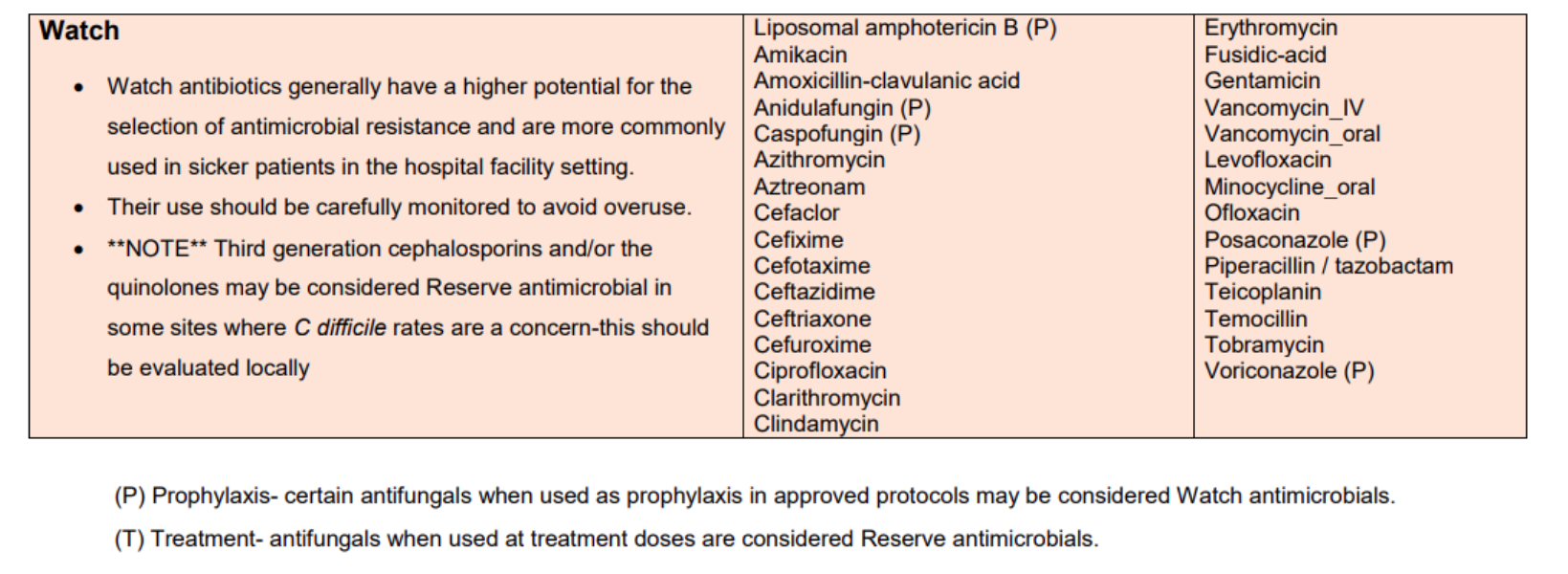
Table 4. List of LH Reserve Antimicrobials (same as HSE AMRIC List).
S/ S*/ R: Interpreting Susceptibility Results
- S - Susceptible, standard dosing regimen: A microorganism is categorised as "Susceptible, standard dosing regimen", when there is a high likelihood of therapeutic success using a standard dosing regimen of the agent.
-
S* – Susceptible, increased exposure 1 : A microorganism is categorised as "Susceptible, Increased exposure*" when there is a high likelihood of therapeutic success because exposure to the agent is increased by adjusting the dosing regimen or by its concentration at the site of infection.
- R - Resistant: A microorganism is categorised as "Resistant" when there is a high likelihood of therapeutic failure even when there is increased exposure.
Summary of changes from previous versions
Dec 2025
Tile 9, Obstetrics and Gynaecology: Sepsis 6+1 For Maternity Patients section updated- new image on Fluid resuscitation algorithm added based on updated HSE National Clinical Guideline on Sepsis Management for Adults (including Maternity), Version 2, 2025.
Nov 2025
Tile 1: Notifiable Diseases - Links in this section updated.
Tile 5, Sepsis Management and Tile 9, Obstetrics and Gynaecology: Sepsis 6 Bundle, Maternity Sepsis 6+1 Bundle, images of Sepsis Screening Tools and references updated based on updated HSE National Clinical Guideline on Sepsis Management for Adults (including Maternity), Version 2, 2025.
Oct 2025
Tile 3: Adult Treatment Guidelines - Skin and Soft Tissue Infections- Cellulitis Severe
- NON-immediate-onset and NON-severe Penicillin Hypersensitivity Empiric IV to PO switch changed from Cefaclor LA to Cef-AL-exin.
Sep 2025
Tile13: Neonatal Guidelines - Neonatal Antimicrobial IV Monographs:
Neonatal - Gentamicin IV updated to unlicensed FK brand, which must be diluted before use (shortage of licensed product)
Neonatal - VZIG IV new monograph.
Aug 2025
Tile 1: General Information - 'Restricted' Antimicrobials section changed to 'Reserve' Antimicrobials. Information on access, watch and reserve antimicrobials based on HSE AMRIC Reserve Antimicrobials National Policy, Dec 2024.
Tile 7: Renal Dosing (Adults) - Chronic Kidney Disease - Cef-AZ-olin monograph updated as per Renal Drug Database, Aug 2025.
Jul 2025
Tile 1: General Infomation:
- New subsection added on S/S*/R : Interpreting Susceptibility Results
Tile 10: IV to Oral Switch Therapy- Appropriate IV to Oral Switch Options:
- Cefuroxime oral switch recommendations for urinary tract infections and other infections updated.
Tile 3: Adult Treatment Guidelines - Urinary Tract Infections: Pyelonephritis/ Urosepsis/Upper UTI:
- 'IV to PO switch' word linked to IV to Oral Switch Therapy section.
May 2025
Tile 3: Adult Treatment Guidelines - Respiratory Tract Infections:
- COVID-19 patients NOT requiring oxygen: Updated with recommended dose for patients with renal impairment. Packaging picture replaced with picture of new packaging and example of kardex prescription removed.
Mar 2025
Tile 3: Adult Treatment Guidelines - Respiratory Tract Infections:
- COVID-19 patients NOT requiring oxygen: Guideline updated based on HSE updated guideline Jan 2025. Paxlovid to be considered only for selected severely immunocompromised adult patients who meet the licensed indications for Paxlovid.
- Pneumocytis jirovecii Pneumonia: New guideline.
Jan 2025
Tile 3: Adult Treatment Guidelines - Malaria - update to advice on sending laboratory specimens for diagnosis as per Consultant Microbiologist.
Tile 3: Adult Treatment Guidelines - Genital Infections - Pelvic Inflammatory Disease - added options for treatment during pregnancy to this section.
Tile 3: Adult Treatment Guidelines - Genital Infections - added link to HSE Antibiotic Prescribing Guidelines for Genital Conditions (same as in Tile 12 STIs).
Tile 9: Obstetrics and Gynaecology:
- Layout reformatted to improve ease of access to information
- Sepsis 6+1 for maternity patients - added images of national sepsis forms for maternity
- Section on GBS Antimicrobial Resistance deleted as relevant information is now included in the GBS intrapartum prophylaxis section
-
Antenatal -
- Added images of national sepsis forms for maternity
- Chorioamnionitis - choice of cephalosporin changed from cef-TRI-axone to cef-UR-oxime as per Rotunda Guidelines and HSE National Guidelines
- Chorioamnionitis - empiric choice for GBS cover for women with immediate-onset or severe penicillin hypersensitivity changed from clindamycin to vancomycin
- Influenza - indications for oseltamivir prophylaxis changed from all close contacts who are pregnant to individual risk assessment
- Listeriosis/septic miscarriage - added to contact clinical microbiologist for advice if concern for CNS infection
- LRTIs - choice of macrolide updated to azithromycin for all trimesters of pregnancy as per Rotunda Guidelines
- LRTIs - severe - first line antimicrobial changed from co-amoxiclav to cef-UR-oxime as per Rotunda Guidelines
- LRTIs - severe - choice of antimicrobials for severe penicillin hypersensitivity - updated to contact clinical microbiologist for advice
- LRTIs - duration of treatment for mild LRTI reduced from 7 days to 5 days and for moderate to severe LRTI from 7 to 10 days to 7 days
- Malaria - removed reference to lack of availability of quinine IV as this has been the case for many years now
- Malaria - update to advice on sending laboratory specimens for diagnosis as per Consultant Microbiologist
- Meningitis - added advice that Cef-TRI-axone should be administered first, followed by Amoxicillin followed by Vancomycin
- Meningitis - added advice to consider dexamethasone, discuss with senior obstetrician
- Tonsillitis - new section added for patients with non-immediate-onset and non-severe penicillin hypersensitivity - cef-AL-exin PO recommended.
- Tonsillitis - choice of macrolide updated to azithromycin for all trimesters of pregnancy as per Rotunda Guidelines
- Tonsillitis - duration changed from 10 days to 5 days for standard therapy with a recommendation to treat for 10 days if scarlet fever suspected or confirmed
- Vulvovaginal candidiasis - dose of canesten pessary increased from 200mg to 500mg nocte for up to 7 nights as per Rotunda Guidelines and BASHH Guidelines
-
GBS IAP -
- Indications for IAP added - image of relevant algorithm from HSE National Guidelines on Prevention of Early-Onset GBS, 2023
- New recommendation for women with immediate-onset or severe penicillin hypersensitivity to perform GBS screen at 35 to 37 weeks so that GBS susceptibility profile is available if IAP indicated
- Empiric choice for GBS IAP for women with immediate-onset or severe penicillin hypersensitivity changed from clindamycin to vancomycin
-
Pyrexia in Labour -
- For first line antimicrobials, added to switch from benzylpenicillin/gentamicin/metronidazole to co-amoxiclav/gentamicin following delivery as per Rotunda Guidelines
- Empiric choice for GBS cover for women with immediate-onset or severe penicillin hypersensitivity changed from clindamycin to vancomycin
-
Postnatal -
- Indications for moderate to severe postnatal infection updated to include source unknown
- Mastitis - mild - dose of flucloxacillin updated to 1g QDS PO, dose of cef-AL-exin increased to 1g QDS PO
- Mastitis - changed title from 'severe' to 'moderate to severe'
- Nipple thrush and ductal candidiasis - updated that fluconzole should only be considered after senior clinician review, dose and duration of fluconazole increased as per Rotunda hospital guidelines
- Urinary tract infections - new section - contains link to adult treatment guidelines for urinary tract infection
- Pelvic Inflammatory Disease - content replaced with link to treatment of Pelvic Inflammatory Disease in Tile 3
- Sepsis post-medical TOP - new section
- Medicines Information in Pregnancy and Breast-Feeding section - references updated
- References updated.
Tile 12: STIs/Genital Conditions - added link to treatment of Pelvic Inflammatory Disease in Tile 3.
Dec 2024
Tile 1: Updated contact details.
Nov 2024
Tile 3: Adult Treatment Guidelines- Head and Neck Infections- Orbital cellulitis First line Antimicrobials options updated with addition of Flucloxacillin 2g QDS IV, and to replace flucloxacillin with vancomycin if history of MRSA colonisation. Non-immediate-onset Penicillin hypersensitivity treatment options updated with addition of Vancomycin.
Tile 3: Adult Treatment Guidelines - Respiratory Tract Infections - Aspiration pneumonia, hospital-acquired and Hospital-acquired pneumonia: Aztreonam no longer in short supply, replaced (with vancomycin) for treatment of patients with penicillin hypersensitivity.
Tile 3: Adult Treatment Guidelines - Gastrointestinal Infections - CDI: Instructions for administration of ORAL vancomycin added.
Tile 11: Gent, Vanc, Amik (Adults) - Adult Gentamicin and Vancomycin sections updated with the link to KDIGO AKI Definition.
Tile 9: Obstetrics and Gynaecology - Entire section updated relating to Vancomycin, updated with addition of loading dose of 25mg/kg (max 2g) before 15mg/kg BD maintenance doses
Tile 9: Obstetrics and Gynaecology - Peripartum Infections/Prophylaxis-Obstetrics-Intrapartum GBS Prophylaxis: Vancomycin Infusion dose changed from 15mg/kg BD to 20mg/kg TDS, maximum single dose 2g until delivery.
Oct 2024
Tile 3: Adult Treatment Guidelines - CNS Infections - Encephalitis: Added link to app calculator to calculate obese dosing weight for aciclovir if needed.
Tiles 3, 7 and 11 - updated gentamicin/vancomycin calculator to version 3. Main changes to the calculator:
- Addition of dosing recommendations for patients with acute kidney injury
- Removal of tick box for 'urine output < 500mL per day'
- Removal of gentamicin dose of 1.5mg/kg - lowest dose depending on renal function now 3mg/kg STAT
- Removal of the restriction on giving a loading dose of vancomycin to pregnant patients, although loading dose capped at 2g in pregnancy.
Tile 11 - updated Adult Once Daily Gentamicin Guideline to version 5. Main changes to the guideline:
- AKI renal dosing added as per recommendations from Critical Illness, Medicines Complete, April 2024
- CKD renal dosing: Removed 1.5mg/kg STAT as dose for CrCl < 10mL/min, replaced with 3mg/kg STAT for CrCl < 50mL/min in all cases
- Added to each dose recommendation ‘N.B. Wait for trough level result before re-dosing if renal impairment’.
- Removed ‘If anuric (output < 500mL/day), treat as CrCl < 10mL/min’.
- For advice on action when trough level > 1mg/L, removed ‘reduce dose by 1 – 2 mg/kg if restarting. Discuss with microbiologist or pharmacist if needed’.
Tile 11 - updated Adult Vancomycin Guideline to version 6. Main changes to the guideline:
- Removal of instruction not to give loading dose in pregnancy – now all patients are to be given the loading dose, although loading dose capped at 2g in pregnancy.
- AKI renal dosing added as per recommendations from Critical Illness, Medicines Complete, April 2024
- Removed ‘If anuric (output < 500mL/day), treat as CrCl < 10mL/min’.
- For advice on action when trough level 10 – 15mg/L, changed ‘Give lower dose more frequently OR increase each dose by 250mg’ to ‘Calculate total daily dose and divide into more frequent doses, e.g. switch 1.5g BD to 1g TDS (preferred) OR increase each dose by 250mg’.
- For advice on action when trough level > 25mg/L, changed ‘ Hold ALL doses until level is repeated next morning and result obtained. If < 20mg/L, restart with lower dose at same frequency, e.g. reduce each dose by 500mg’ to ‘ HOLD vancomycin dose(s). Usually HOLD until level repeated and result obtained next morning. When level < 20mg/L, restart with lower dose at same frequency, i.e. reduce each dose by 500mg. N.B. - if patient has required a high dose to reach therapeutic level, they are likely to clear vancomycin quickly and it may be appropriate to hold a single dose only. Contact pharmacist for advice if needed’.
Aug 2024
Tile 13- Neonatal Guidelines- Neonatal Medical Prophylaxis-Prevention of Perinatal Transmission of HIV, HBV, HCV and Syphilis-Rainbow Clinic Guidelines document location changed from T Drive to HCI-Knowledge.
Tile 13- Tile 13- Neonatal Guidelines- Neonatal Medical Prophylaxis- Respiratory Synctial Virus Prophylaxis: Manual of Neonatal Guidelines removed, Standard Operating Procedures for Referral Process for Synagis (Palivizumab) administration in the community and administration of Synagis in the Neonatal Intensive Care Unit in Our Lady of Lourdes Hospital Drogheda document location added.
Jul 2024
Tile 11 - Gent/Vanc/Amik - Adult Amikacin Guideline - doses changed from 'daily' to 'once daily'.
Jul 2024
Tile 1: Prescribing principles for adults and paediatrics merged into one section.
Tile 5: Sepsis Management - sections on sepsis management for adults, maternity and paediatrics now in this section. Maternity moved from Tile 9 Obs & Gynae, Paediatrics moved from Tile 12 Paediatrics. Paediatric sepsis management guideline changed from general sepsis wording to reference HSE national clinical guideline on sepsis for paediatrics and paediatric sepsis 6 picture added.
Tile 6: Title changed from 'Adult Surgical Prophylaxis' changed to 'Surgical Prophylaxis'. Surgical prophylaxis for Paediatrics moved from Tile 12 to Tile 6. Principles of surgical prophlylaxis for both adults and paediatrics merged into one section. Section on doses of each agent for surgical prophylaxis added for both adults (new) and paediatrics (moved from Tile 12).
Tile 10: IV to Oral Switch Therapy - this section is now applicable to both adults and paediatrics. Section on 'Paediatric IV to PO switch options' removed in line with CHI guidelines - this detailed section is not appropriate for an empiric antimicrobial guidelines app, clinical microbiology to be contacted for advice if required.
Tile 12: Order of tiles changed - Tile 12 is now for STI Infections, Tile 13 is for Neonatal Guidelines and Tile 14 is for Paediatric Guidelines. Previously, Tile 12 contained both Neonatal and Paediatrics Guidelines.
Tile 13: Neonatal Guidelines - monographs for neonatal meningitis (community-acquired), neonatal sepsis community-acquired and neonatal urinary tract infection updated to refer to guideline for 'Paediatrics - sepsis in babies < 8 weeks old'.
Tile 14: Added that users should refer to CHI antimicrobial guidelines app for recommended paediatric antibiotic doses instead of BNFc.
Tile 14: Paediatric Guidelines - Paediatric Antimicrobial Prescribing Principles moved to Tile 1 as above, Paediatric Sepsis Management moved to Tile 5 as above, Paediatric Surgical Prophylaxis moved to Tile 6 as above, Paediatric IV to PO switch section moved to Tile 10 as above.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Central Nervous Infections:
- See separate monograph for Sepsis (previously both sections included in the one monograph).
- Monograph updated as per CHI for children <= 8 weeks to dual therapy with cef-O-taxime IV plus amoxicillin IV as standard therapy, consider addition of gentamicin (previously standard therapy), vancomycin, aciclovir and clindamycin based on risk factors. Removal of recommendation that cef-O-taxime may be switched to cef-TRI-axone once daily after 24 hours once diagnosis is clear and/or patient has stabilised as this recommendation is no longer in CHI guideline. Addition of comments as per CHI Guideline. Addition as per LH Consultant Microbiologist of advice to contact microbiology if hx ESBL or other resistant organism in the mother or baby.
- Monograph updated as per CHI for children > 8 weeks to cef-O-taxime IV as standard therapy, consider addition of gentamicin (previously standard therapy), vancomycin, aciclovir and clindamycin based on risk factors. Removal of recommendation that cef-O-taxime may be switched to cef-TRI-axone once daily after 24 hours once diagnosis is clear and/or patient has stabilised as this recommendation is no longer in CHI guideline. Addition of comments as per CHI Guideline. Addition as per LH Consultant Microbiologist of advice to contact microbiology if hx ESBL or other resistant organism in the mother or baby.
- Durations of treatment for meningitis kept the same as HPSC Guidelines 2016.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Central Nervous Infections: Ventriculitis with CNS shunt - monograph removed as not considered relevant for OLOL.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: ENT Infections: Dental Infections and ENT Infections are now separate sections.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Dental Infections: Penicillin allergy treatment options added, section for severe infection added, comments as per CHI guideline added, link to HSE Dental Guidelines at www.antibioticprescribing.ie added.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: ENT Infections: Cervical Lymphadenitis: added Cefazolin IV as an alternative to flucloxacillin IV plus clindamycin PO; duration for mild to moderate reduced from 14 to 7 days; duration for severe changed from 2 to 3 weeks to ‘as per micro’, IV to PO switch is when clinically appropriate, added to contact ENT if suppuration present. Added cefalexin PO as an appropriate oral switch from cefazolin IV.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: ENT Infections: Peritonsillar abscess: Duration reduced from 14 to 10 – 14 days, also duration depends on response and drainage; added to consider discontinuing clindamycin after 3 to 5 days.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: ENT Infections: Tracheitis – Bacterial: Duration reduced from 10 to 14 days to 7 to 10 days.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: ENT Infections: Acute mastoiditis: Duration reduced from 28 days to 14 days. Added to contact micro if MRSA or Pseudomonal cover required, recommended options for these cases removed.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: ENT Infections: Chronic mastoiditis: Duration reduced from 28 days to 14 days in CHI, change to ‘individual case-by-case basis’ in OLOL.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: ENT Infections: Otitis Externa: Changed to first line topical Ciprofloxacin ear drops or if systemic, Rx flucloxacillin or cefalexin PO.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: ENT Infections: Otitis Media – Acute: Duration reduced from 10 days to 5 – 7 days.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: ENT Infections: Pharyngitis: Duration reduced from 10 to 5 days for phenoxymethylpenicillin or amoxicillin, however if severe/scarlet fever (GAS (+) throat swab) duration of 10 days. Switch from Azithromycin for penicillin allergic patients to either cef-AL-exin or clarithromycin depending on the nature of the allergy as per HSE Guidelines on www.antibioticprescribing.ie. Also added comments that antibiotics make little difference to symptoms.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: ENT Infections: Sinusitis: Duration reduced from 10 – 14 days to 5 days.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Respiratory Tract Infections: Aspiration pneumonia, added option for penicillin allergic patients: co-trimoxazole IV plus metronidazole. Duration reduced from 7 days to 5 days as per CHI Guidelines.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Respiratory Tract Infections: CAP, child <= 8 weeks: Content updated as per sepsis monograph updates. Duration reduced from 2 - 3 weeks to 5 days as per CHI Guidelines.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Respiratory Tract Infections: CAP, child > 8 weeks: Duration of treatment reduced as per CHI Guidelines from 7 - 14 days to 5 days for mild to moderate pneumonia and from 14 - 21 days to 5 - 10 days for complicated pneumonia.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Respiratory Tract Infections: Pertussis: Duration of treatment for Azithromycin reduced as per CHI Guidelines (and UKHSA Pertussis Guidelines 2024) from 5 days to 3 days. Note this is an unlicensed indication.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Sepsis - new section, previously monograph combined with meningitis. Antimicrobial recommendations / changes as per CNS - meningitis monographs above.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Skin, Soft Tissue and Surgical Wound Infections: Animal or human bites – Added recommendations for prophylaxis: 1. No prophylaxis if skin is unbroken, 2. Consider prophylaxis is skin is broken but not drawn blood if risk factors, 3. Offer prophylaxis if skin is broken and drawn blood. Co-amoxiclav x 3 days recommended for prophylaxis. Treatment of infected bite remains as co-amoxiclav for 5 days, add option for penicillin allergy, co-trimoxazole PO.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Skin, Soft Tissue and Surgical Wound Infections: Burns - Changed from addition of gentamicin to pip/tazobactam if late-onset infection > 5 days post-hospitalisation. Added comments to send swabs and review based on C&S.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Skin, Soft Tissue and Surgical Wound Infections: Cellulitis – added section for mild to moderate cases with oral flucloxacillin or cefalexin recommended; reduced duration from 10 days to 5 to 7 days, with a note that courses can be extended up to 14 days if clinically indicated.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Skin, Soft Tissue and Surgical Wound Infections: Impetigo – reduced duration from 10 to 5 – 7 days.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Skin, Soft Tissue and Surgical Wound Infections: Severe Skin and Soft Tissue Infection with Systemic Illness: e.g.Necrotising fasciitis, Toxic shock-like illness – replaced content of monograph with advice to contact microbiology urgently. Monograph for synergistic gangrene removed.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Skin, Soft Tissue and Surgical Wound Infections: SSI – if contaminated wound or slow to respond or Gram negative organism suspected, change from addition of gentamicin to pip/tazobactam instead. IF known MRSA, change from addition of vancomycin to advice to contact microbiology.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Urinary Tract Infections: Treatment of UTI in children < 2 months updated to amoxicillin IV plus cef-O-taxime IV +/- gentamicin IV based on risk factors as per CHI update for paediatric sepsis treatment in children < 8 weeks.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Cardiac infections, Tuberculosis treatment and prophylaxis sections removed.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Febrile Neutropenia section updated with 'refer to CHI app for guidance'.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Fungal Infections section updated with 'refer to CHI app for guidance'.
Tile 14: Paediatric Guidelines: Paediatric Empiric Treatment Guidelines: Gastrointestinal infections - monographs for Campylobacter, Salmonellosis, Shigellosis and C. difficile infection removed as these are directed rather than empiric guidelines.
Tile 14: Paediatric Guideliness: Paediatric Empiric Treatment Guidelines: Respiratory tract infections: monographs on empyema and pneumonia with bullae removed. Monograph for infective exacerbation of cystic fibrosis updated with 'refer to CHI app for guidance'. Monograph for pneumonia in hospital inpatients monograph updated with advice to consult microbiology.
Tile 14: Paediatric Guidelines: Paediatric Gentamicin and Vancomycin Guidelines - reference updated to CHI 2020, content the same.
May 2024
Tile 6: Adult Surgical Prophylaxis: Obstetrics & Gynaecology Surgical Prophylaixis: Updated with antibiotic choice for Ureteric stenting during Obstetrics & Gynaecology procedures.
Tile 6: Adult Surgical Prophylaxis: Urogenital Surgery Prophylaxis: Updated with Surgical Antibiotic prophylaxis in Ureteric stenting.
Tile 13: Sexual Transmitted Infections/ Genital Conditions: New section with link to access treatment options of Genital conditions.
Tile 9: Obstetrics and Gynaecology: Other Infections in Pregnancy: Obstetrics-Varicella Zoster Virus (VZV)-Post Exposure Prophylaixs during Pregnancy: Updated with Valaciclovir PO to be used as 1st line option where appropriate.
Tile 6: Adult Surgical Prophylaxis: Principles of Surgical Prophylaxis: Number of doses of surgical prophylaxis: section reworded.
Tile 6: Adult Surgical Prophylaxis: Gentamicin renal dose reworded across section.
Tile 6: Adult Surgical Prophylaxis: Abdominal, Gastrointestinal and General Surgery Prophylaxis: Appendicectomy: post-operative antimicrobial therapy changed from 5-7 days to 3-7 days.
Tile 4: Adult Medical Prophylaxis: Endocarditis Prophylaxis: Section updated with link to most recent guideline.
Tile 1: General Information: Restricted Antimicrobials: Aztreonam removed, daptomycin and ambisome added. Also changed location of LH Restricted Antibiotics Policy to HCI-Knowledge-RCSI HG.
Mar 2024
Tile 7: Renal dosing of antimicrobials: Added information with regard to dosing in AKI from 'Critical Illness' and BNF. Tables of renal dose adjustments moved to a section titled 'CKD' and section specifies that the tables apply to CKD only and not for patients with AKI or on dialysis.
Feb 2024
Guidelines updated in relevant sections with regards to ciprofloxacin and metronidazole PO to be considered from outset and the IV route chosen only where oral route is not feasible.
Jan 2024
Tiles 3, 9, 10: Guidelines updated due to shortage of clarithromycin LA 1g once daily PO. Switch from Clarithromycin LA 1g once daily PO to Clarithromycin 500mg BD immediate-relaese PO for adult and obstetric respiratory tract infections, adult ENT infections, adult neutropenic sepsis guidelines and in the IV to PO switch section.
Dec 2023
Tile 3 and Tile 9: Routes of administration for clarithromycin reworded from PO or IV (excellent oral bioavailability) to PO (or IV only where oral route is not feasible - excellent oral bioavailability). Note that clarithromycin immediate release tablets in short supply removed as Klacid LA now routinely stocked and recommended.
Tile 3: Respiratory Tract Infections: Routes of administration for Amoxicillin and Metronidazole reworded from PO or IV (excellent oral bioavailability) to PO (or IV only where oral route is not feasible - excellent oral bioavailability).
Tile 3: ENT Infections: Routes of administration for Ciprofloxacin reworded from PO or IV (excellent oral bioavailability) to PO (or IV only where oral route is not feasible - excellent oral bioavailability).
Sep 2023
Tile 3: Hospital-acquired pneumonia and aspiration pneumonia - clarified that patients should be treated for this indication if they have been in hospital in previous 6 weeks and not only if they had IV antibiotics in the last 6 weeks, e.g. during a previous admission.
Tiles 3, 9, 10: Guidelines updated due to shortage of clarithromycin 500mg immediate release tablets. Switch from Clarithromycin 500mg BD PO to Clarithromycin LA 1g once daily PO for adult and obstetric respiratory tract infections, adult ENT infections, adult neutropenic sepsis guidelines and in the IV to PO switch section. The following guidelines were NOT switched and will still recommend clarithromycin immediate release tablets or liquid: H. pylori treatment, paediatric guidelines, patients with swallowing difficulties or who receive medicines via feeding tubes.
Tile 7: Renal dosing updated - minor changes based on review of Renal Drug Database recommendations. Aciclovir IV and PO updated, Aztreonam updated with local LH practice, Cefuroxime PO removed as no longer stocked in LH, Daptomycin added.
Tile 12: Paediatric and Neonatal empiric treatment guidelines for conjunctivitis updated - previous warning on use of chloramphenicol eye drops in patients less than 2 years has been reviewed and revoked, therefore empiric treatment returned to chloramphenicol eye drops as a first line agent.
April 2023
Throughout: References updated.
Tile 1: Contact details updated.
Tile 3:
- Neutropenic sepsis - definition removed from guideline.
- Scrotal abscess - empiric IV to oral switch option removed as oral switch depends on many factors.
- Healthcare-Associated Intra-abdominal infections - title changed to Hospital-Acquired Intra-Abdominal Infections
- Community-Acquired and Hospital-Acquired Intra-abdominal infectons - removed colonic perforation as an indication for possible anti-fungal therapy.
- Catheter-associated bacteriuria - "suprapubic pain" changed to "flank pain".
Tile 6: CDC/NHSN Definitions of Surgical Site Infection updated as per publication Jan 2023.
March 2023
Tile 3: Adult Treatment Guidelines
-
Bone and Joint Infections:
- New section for "Discitis / Vertebral Osteomyelitis / Vertebral Abscess"
- Changed non-immediate penicillin allergy guideline from cefuroxime IV 1.5g TDS to cefazolin IV 2g TDS for Acute Osteomyelitis and Septic arthritis in Native Joint sections
- Central Nervous System Infections - Meningitis: Removed the word "Consider" from the recommendation to add Dexamethasone.
- Intra-abdominal Infections - Appendicitis: Duration changed from “5-7 days” to “Uncomplicated: Post-op antibiotics not indicated. Complicated: 5-7 days” in line with Beaumont guidelines and to match current recommendations in surgical prophylaxis section.
-
Respiratory Tract Infections:
- Community Acquired Pnuemonia (CAP): Changed Amoxicillin dose to 1g TDS PO for mild CAP. Removed definition of CAP from each of the 3 CAP sections.
- COVID-19 (SARS-CoV-2) Infection: New section.
-
Skin and Soft Tissue Infections:
- Diabetic Foot Ulcer: Change mild/ moderate (deep /superficial) Flucloxacillin dose to 1g QDS PO rather than 500mg- 1g PO in line with recent cellulitis change
- Necrotising Skin and Soft Tissue Infections: Changed Piptazobactam frequency to QDS rather than TDS
- Changed non-immediate penicillin allergy guideline from cefuroxime IV 1.5g TDS to cefazolin IV 2g TDS for Cellulitis and PVC Infection sections.
- Vascular Catheter Infections: PVC - removed empiric IV to PO switch options.
- Added requirement for MRSA screen on admission for orthopaedic patients as per current IPC policy
- Added chlorhexidine to mupirocin for MRSA decolonisation in line with current protocol
Tile 11: Adult Gentamicin Guideline (updated to Version 4, 2023)
December 2022
All sections: Minor wording changes, diagnostic tests updated (e.g. swab in red-top VTM rather than pink-top swab for respiratory viral PCR), links to other sections of app updated
Tile 9: Obstetrics & Gynaecology: Other infections in pregnancy: Obstetrics- Meningitis: Comment re shortage of amoxicillin Mar 2020 removed
Tile 4: Adult Medical Prophylaxis: Meningococcal Prophylaxis for Contacts: Public Health phone number updated
Tile 12: Neonatal and Paediatric Guidelines: Neonatal Empiric Treatment Guidelines: Neonatal Sepsis Early Onset
- Link to early onset sepsis risk assessment calculator inserted
Tile 3: Adult Treatment Guidelines: Skin and Soft Tissue Infections: Cellulitis- Mild to Moderate
- Flucloxacillin dose changed from '500mg to 1g QDS PO' to '1g QDS PO'
Tile 3: Adult Treatment Guidelines: Voriconazole Prescribing Aid
-
Type of sample container for Voriconazole trough level check
Tile 3: Adult Treatment Guidelines: Gastrointestinal Infections: Clostridiodes difficile Infection (CDI)
- Vancomycin PO dose increased from '125mg' to '500mg' QDS in Severe Complicated CDI
Tile 3: Adult Treatment Guidelines: Respiratory Tract Infections
- Influenza testing recommendations updated with new swab and in-house testing details. Influenza care pathway removed as obsolete.
June 2022
Tile 9: Obstetrics & Gynaecology
- New subsection added with "Sepsis 6 + 1" protocol from 2021 national guidelines on Sepsis Management for Adults including Maternity.
Tile 5: Sepsis Management
- Updated to reflect 2021 national guidelines on Sepsis Management for Adults including Maternity and 2021 updated Surviving Sepsis Campaign guidelines.
May 2022
Tile 3: Respiratory Tract Infections- Community Acquired Pneumonia (CAP) MODERATE CURB-65 Score 2
- Amoxicillin 1g TDS PO or IV (previously Amoxicillin PO or Co-amoxiclav IV) Plus Clarithromycin 500mg BD PO or IV ( Amoxicillin IV was previously in short-supply but now available)
Tile 6: Adult Surgical Prophylaxis: Obstetrics and Gynaecology Surgical Prophylaxis
- Gynae Ablation procedure is updated with Surgical Antibiotic Prophylaxis is not recommended (previously not on guideline)
March 2022
Tile 11: Adult Vancomycin Guideline and Vancomycin Calculator updated with ' Do not use in ICU/HDU to calculate dosing for Vancomycin Continuous Infusion'
Tile 6: Adult Surgical Prophylaxis: Urogenital Prophylaxis: Transurethral Resection of Bladder Tumour
- Gentamicin single dose (previously no prophylaxis recommended)
February 2022
Tile 1: General Information: Contact Information
- Microbiology registrar contact information updated
Tile 3: Gastrointestinal Infections: Clostridioides difficile infection (CDI):
- First-line antimicrobial for mild/moderate first episode changed from metronidazole to vancomycin 125mg QDS PO and duration changed to 10 days.
- Vancomycin dose for severe complicated CDI changed from 500mg QDS to 125mg QDS.
August 2021
Tile 6: Adult Surgical Prophylaxis: Obstetrics and Gynaecology Surgical Prophylaxis:
- Table added on prophylaxis for Assisted/ Operative Vaginal Delivery
- Update to HSG/ HYCOSY prophylaxis recommendation- no prophylaxis required if STI screen negative
May 2021
Tile 12: Neonatal Guidelines: Neonatal Antimicrobial IV Monographs - individual monographs updated, all changes listed on each monograph revision history.
April 2021
Tile 11: Adult Vancomycin Guideline and Vancomycin Calculator updated with new max loading dose of 3g (max maintenance dose remains at 2g), simplified renal dosing and updated advice on action to take when trough level is out of range.
Feb 2021
Tile 3: Urinary Tract Infections: Titles of monographs reworded to include "UTI".
Tile 6: Addition of surgical prophylaxis recommendation for HSG, HYCOSY
Tile 7: Correction of renal dose monograph for cef-AL-exin based on recommendation from John Hopkins ABX Guide.
Tile 9: IV to PO switch options: Added that there is no empiric oral switch from IV pip/tazobactam.
Tile 12: Neonatal and Paediatric Conjunctivitis monographs - chloramphenicol no longer recommended for children less than 2 years.
Tile 12: Included the word "Paediatrics" on each section (where this is not already done) to avoid confusion if using the search function on the app.
Oct 2020
Tile 12: Paediatric Empiric Treatment Guidelines - addition of sentence that PO cef-UR-oxime is not recommended due to low oral bioavailability in sections where IV cef-UR-oxime is part of the recommended initial treatment regimen (ENT, appendicitis, pneumonia, urinary tract infections).
Sep 2020
Tile 1: Contact details updated.
Tile 3: Shortage of IV Amoxicillin Sep 2020 - treatment monographs for dental abscess and moderate CAP updated to reflect this. Limited stocks of amoxicillin IV reserved for remaining indications.
Tile 9: Shortage of IV Amoxicillin Sep 2020 - treatment monograph for PPROM updated to reflect this. Limited stocks of amoxicillin IV reserved for remaining indications.
Jul 2020
Tile 6: Adult surgical prophylaxis
- CDC/NHSN Definition of SSI added
- Gynaecology Procedures listed in greater detail and SAP recommendations updated accordingly, main reference ACOG Guidelines 2019.
Tile 9: Obstetrics and Gynaecology
- Monographs on STIs during pregnancy removed as this is available in the GUM Clinic Guidelines. Section on PID retained.
- Useful references for checking safety of medicines in pregnancy and breastfeeding updated. Information on FDA categories removed.
Jul 2020
Tile 1: Restricted Antimicrobials - Fidaxomicin added to the list.
Tile 3: All subsections
- Change in terminology: “delayed- onset” changed to “non-immediate-onset” in reference to penicillin hypersensitivity, “Consultant Microbiologist” to “Clinical Microbiologist”.
- Standardisation of information on precautions when gentamicin, vancomycin, quinolones or clarithromycin recommended.
- Guideline reworded as needed to increase clarity and consistency.
- Empiric IV to oral switch option added to each monograph when appropriate.
Tile 3: Bone and Joint Infections
- Added that vancomycin should be substituted for flucloxacillin if history of MRSA colonisation.
- Duration sections reworded.
- Fusidic acid interaction with statins removed as fusidic acid no longer used in practice in LH due to AMR.
- Septic arthritis - PJI: Information on rifampicin reworded.
Tile 3: Vulvovaginal candidiasis
- Treatment options updated as per BASHH Guidelines 2019.
- Addition to refer patients with recurrent candida to GU/ID service.
- Removal of rifampicin as this is not started with initial empiric regimen and it is more appropriate to be advised as an addition by Clinical Microbiologist if indicated.
- Microbiological investigations, removal of travel history as a reason to consider TB, change of PCR request from specified viruses to “viral PCR”.
- Addition of reminder to reduce dose of aciclovir in renal impairment.
Tile 3: Meningitis
- Addition of maximum 10mg per dose for dexamethasone 0.15mg/kg QDS IV
- Removal of alternatives due to shortage of amoxicillin as remaining stock will be reserved for certain indications only including meningitis.
Tile 3: ENT Infections
- Section reformatted. Increase PO doses to higher end of PO range as per usual practice and GP ENT Guidelines www.antibioticprescribing.ie
- Removal of section on management of SSI following cochlear implant surgery as this procedure is not performed in LH
- Quinsy: Addition that mycobacterial staining and TB culture may be indicated
- Acute Otitis Externa: Removal of paediatric doses from adult guideline.
- Acute Otitis Media: Removal of information on management if cochlear implant in situ as procedure not performed in LH, specialist advice recommended.
- Acute mastoiditis: separation into separate monographs for uncomplicated and complicated acute mastoiditis.
- Acute mastoiditis – uncomplicated: Addition of options for penicillin hypersensitivity.
- Acute mastoiditis – complicated: Third generation cephalosporin switched from cef-O-taxime to cef-TRI-axone, addition of metronidazole.
- Acute rhinosinusitis: Duration reduced from 7 to 10 days to 5 days as per GP ENT Guidelines; Penicillin allergy alternatives updated as per GP Guidelines, previously clarithromycin only, now clarithromycin or doxycycline.
- Dental abscess: Switch from initial PO to IV therapy for severe infection.
Tile 3: Gastrointestinal Infections
- Clostridium difficile renamed as Clostridioides difficile
- C. difficile: Removal of reference to proforma letter as no longer sent in practice.
Tile 3: Genital Tract Infections
- Acute epididymo-orchitis: When STI likely/possible, switch from cef-TRI-axone 1g IM for all patients to add if N. gonorrhoeae strongly suspected as per GU/ID recommendation. Addition to STI screen: Urine for M. genitalium PCR, serum for HIV, hepatitis and syphilis.
- PID: Removal of information on empiric treatment of patient’s partner as this is better managed by the GUM clinic. Monograph recommends GUM clinic referral. Addition to STI screen: Change from endocervical to vulvovaginal swab, request urine or swab for M. genitalium PCR, serum for HIV, hepatitis and syphilis.
Tile 3: Hepatobiliary and Pancreatic Infections
- Acute pancreatitis: Change from pip/taz to pip/taz +/- gentamicin if clinical sepsis.
- SBP: Expansion of detail on specimen of ascitic fluid required for white cell count and C&S.
Tile 3: Intra-abdominal Infections
- Community-acquired intra-abdominal infections: Divided into two sections, empiric IV to oral switch provided for appendicitis but not for other infections listed.
- Perianal abscess and ischiorectal abscess: New section
Tile 3: Respiratory Tract Infections
- Aspiration pneumonia (community or hospital acquired): Reduce duration from 7 days to 5 to 7 days.
- Aspiration pneumonia, hospital acquired: Change of empiric choice from ciprofloxacin and metronidazole to aztreonam,vancomycin and metronidazole.
- CAP Moderate: Removal of alternative option due to shortage of amoxicillin IV.
- HAP: Duration reduced from 7 days to 5 to 7 days.
- Respiratory TB: Comment to always contact ID or Respiratory changed to Respiratory only based on current referral structure in LH. Addition of HIV test to Microbiological Investigations.
Tile 3: Skin and Soft Tissue Infections
- Erysipelas: Section deleted
- Bursitis: New section
- Herpes Zoster: Addition of HIV test to Microbiological Investigations
- Necrotising SST: Increase clindamycin to max dose 1.2g QDS IV
- Penetrating trauma: New section
Tile 3: PVC infection: Addition of option if MRSA colonisation.
Tile 4: Adult Medical Prophylaxis
Meningococcal prophylaxis for contacts: Detail expanded.
Variceal Haemorrhage Prophylaxis: Change cef-TRI-axone dose from 2g to 1g daily as per AASLD Guidelines.
Tile 6: Adult Surgical Prophylaxis: TRUS biopsy prophylaxis: Added renal dose amikacin, changed “CPE/CRE” to “CPE”
Tile 10: IV to Oral Switch
- IV to oral switch criteria reworded to “COMS” criteria based on AMS IV to PO switch sticker. Examples of deep-seated and high-risk infections updated as per IV to PO sticker.
- Table of IV to oral switch options: Doses updated, choice of oral cephalosporin following IV cef-UR-oxime updated based on recent AMS memo.
Mar 2020
Tile 1: Contact details updated.
Tile 3: Adult treatment guidelines
- Shortage of Amoxicillin Mar 2020 - treatment monographs for meningitis and moderate CAP updated to reflect this.
- Pip/tazobactam no longer in short supply - treatment monographs updated to remove alternative options when pip/taz not available.
- Oral candidiasis: Dose of fluconazole for moderate to severe infection increased to 200mg once daily as per SPC/Sanford/John Hopkins (previous dose as per BNF).
- Acute epididymo-orchitis: Dose of Cef-TRI-axone increased to 1g IM as per updated BASHH Guidelines for Gonorrohea.
- Acute prostatitis: Duration amended from 28 days to 14 days then review: stop or continue for another 14 days as per NICE Guidelines.
- PID mild to moderate: Dose of Cef-TRI-axone increased to 1g IM as per updated BASHH Guidelines for PID.
- Acute cholangitis: Recommendation changed from pip/taz +/- gentamicin if clinical sepsis to Pip/taz AND Gentamicin.
- SBP: Choice of agent changed to cef-TRI-axone as per Beaumont Hospital guideline update.
- Terminology of “healthcare-associated” pneumonia changed to “hospital-acquired” pneumonia as per ATS Guidelines 2016. Definition of HAP updated as per update from ATS Guidelines 2016 and local amendments as per Beaumont Hospital (recent IV abx = 6/52).
- HAP: Penicillin Hypersensitivity – change from ciprofloxacin and vancomycin to aztreonam and vancomycin to reduce FQ use based on recent CHMP warnings.
- Mild CAP: Duration reduced to 5 days as per NICE Guidelines on CAP and Beaumont update.
- Severe CAP: Duration reduced to 7 days as per NICE Guidelines on CAP and Beaumont update.
- Cellulitis: Change of terminology to “Mild to Moderate” and “Severe” based on Beaumont update. New section added on choice of agent for patients with known or risk factors for MRSA colonisation.
- Diabetic Foot Ulcer, deep with indications for hospitalisation and Diabetic Foot Osteomyelitis: Indications for use of pip/taz instead of co-amoxiclav expanded as per Beaumont update
- References updated
Tile 4: Variceal haemorrhage prophylaxis: Choice of agent changed to cef-TRI-axone as per Beaumont Hospital guideline update.
Tile 9: Obstetrics and Gynaecology
- Shortage of Amoxicillin Mar 2020 - treatment monograph for meningitis in pregnancy updated to reflect this
- Chlamydia in Pregnancy - dose of azithromycin updated based on updated BASHH Guidelines
- Gonorrhoea in Pregnancy - dose of cef-TRI-axone updated based on updated BASHH Guidelines, concomitant azithromycin removed.
Tile 10: IV to PO switch options: Added that PO cef-UR-oxime not recommended due to low oral bioavailability. Oral stepdown from IV cef-UR-oxime updated to cefaclor for respiratory tract infections and as per C&S for UTI.
Nov 2019
Tile 1: Restricted Antimicrobials - Aztreonam added.
Tile 3: Mild and moderate community-acquired pneumonia, IE COPD: Change of duration from 7 days to 5 to 7 days as per updated NICE Guidelines. Also change of duration for IE asthma to 5 to 7 days.
Tile 6: Surgical prophylaxis - surgery performed in the setting of infection: Change to surgical prophylaxis indicated on proceeding to theatre (except gentamicin/vancomycin/teicoplanin) to ensure SAP administered within 60 minutes before incision.
Tile 12: Paediatric Guideline Updates - LH Paediatric Antimicrobial Guideline reviewed based on updated OLCHC-TSCUH Antimicrobial Guidelines 2019 (with kind permission):
- Prescribing Principles: Information on dosing moved to the section on guideline scope, other prescribing principles from LH Adult/Neonatal Antimicrobial Guidelines also incorporated
- Evaluating for antibiotic allergies: OLCHC-TSCUH 2019 Guidelines updated section included.
- IV to PO switch: Minor changes to comments, additional cautions for fluoroquinolones added. PO switch for IV Clarithromycin in OLOL is PO Clarithromycin
- Gentamicin Paediatric Guideline: In first edition of LH Paediatric Antimicrobial Guidelines, LH Gentamicin summary algorithm included only (same information as national paediatric gentamicin guideline, different layout). In this edition, LH summary algorithm and full national 2016 gentamicin guideline included (except for neonatal guidance and removal of sentence re possibility of once daily dosing in endocarditis as this is not undertaken in practice). New renal dosing by OLCHC-TSCUH added (confirmed with OLCHC-TSCUH that GFR measurement is ml/min/1.73m 2 . Gentamicin administration over 30 min in OLOL (20 – 30 min in OLCHC-TSCUH).
- Vancomycin Paediatric Guideilne: Updates as per OLCHC-TSCUH, Addition of a loading dose for certain patients, Information on assessment of renal function, Updated renal dosing, If level 21 -24 mg/L, reduce dose by 10% but do not hold next dose. Other changes: Updated summary algorithm, LH lab processing times included.
- Chronic Osteomyelitis: Rephrased sentence regarding withholding antimicrobial treatment until C&S results are available.
- CNS: Dexamethasone 0.15mg/kg, maximum dose increased from 4mg to 10mg. Max dose 10mg as per BNFc.
- Encephalitis: Aciclovir dosing not included as available in BNFc.
- Campylobacter sepsis/bloodstream infection: Recommendation to consult Microbiology for advice
- CDI: Section updated as per OLCHC-TSCUH (reference IDSA & SHEA Guidelines, 2017)
- Acute appendicitis: Change from empiric co-amoxiclav or amoxicillin/metronidazole/gentamicin to empiric cef-UR-oxime plus metronidazole +/- gentamicin based on review of local E.coli resistance rates in paediatrics.
- Acute appendicitis: Removed advice to give 24 hours of antibiotics for inflamed appendix.
- Malaria: Added dosing example for Riamet, Added dose of Artesunate IV as not available in the BNFc (dose as per WHO/Sanford/JH), Added information on why primaquine is recommended and when to start – wait for result of G6PD deficiency screen. Confirmed with Dr Justin Low, ID Consultant.
- UTI: Change from empiric co-amoxiclav plus gentamicin to empiric cef-UR-oxime +/- gentamicin based on review of local E.coli resistance rates in paediatrics.
- Surgical Prophylaxis: LH local changes made in 2017 compared to 2015 OLCHC-TSCUH Guidelines, further changes in this update - surgical prophylaxis for paediatric urology procedures removed as not undertaken in LH, dose of teicoplanin for prophylaxis updated to include children 1 to 2 months as well as > 2 months.
Sep 2019
Tile 3: Non-falciparum malaria: P. vivax and P. ovale - addition that primaquine can be started in the follow-up OPD appointment once results of G6PD deficiency screen known. Confirmed with Dr Justin Low, ID Consultant.
Aug 2019
Tiles 3, 9 and 12: Malaria for adults, obstetrics and paediatrics – addition of a worked example of Riamet® dosing to aid correct administration.
Jul 2019
Tile 1: Contact details updated.
Tile 3 and Tile 9: Meningitis (for adult and maternity patients) - switch of 3rd generation cephalosporin from cef-O-taxime IV to cef-TRI-axone IV due to convenience of BD dosing regimen for nursing staff.
Tile 3, Tile 9 and Tile 12: Malaria (for adult, maternity and paediatric patients) - updated as Quinine IV no longer available. IV Artesunate is already first line for severe malaria.
Tile 9: The word "Obstetrics" added to the title of Infection Treatment Monographs in the "Obstetrics and Gynaecology" tile in case the search function is used to check treatment recommendations and the user may not realise that the particular section is for obstetric patients. Pelvic Inflammatory Disease - both mild and severe previously included in the Obstetrics section, however mild is not applicable to pregnant patients and is already covered in the Adult Treatment Guidelines Section so removed from the Obstetrics section.
Tile 12: The word "Neonatal" or "Paediatrics" added to the title of Infection Treatment Monographs in the "Neonatal and Paediatrics" tile in case the search function is used to check treatment recommendations and the user may not realise that the particular section is for neonatal or paediatric patients.
Tile 12: Neonatal vancomycin IV monograph updated. All changes are listed at the end of the monograph.
Apr 2019
Tile 1: Contact details updated
Tile 3: Pip/tazobactam quota increased - still less than usual use but higher than previous quota. The following sections have been updated to include a recommendation for use of pip/tazobactam and also an alternative empiric regimen if pip/tazobactam is not available:
acute cholangitis, healthcare-associated intra-abdominal infections, acute pancreatitis, diabetic foot ulcer deep infection or osteomyelitis, necrotising skin and soft tissue infections, neutropenic sepsis, healthcare-associated pneumonia and healthcare-associated aspiration pneumonia.
Tile 3: Influenza Treatment - OLOL Influenza Care Pathway added.
Tile 12: UTI section - information added that nitrofurantoin liquid is not readily available in the community and that advance notice is required for the community pharmacy if possible.
Mar 2019
Tile 1: Contact details updated
Tile 6: Adult Surgical Prophylaxis
- Caution on the risk of anaphylaxis with teicoplanin added.
- References updated.
Tile 9: Obstetrics and Gynaecology
- Section on GBS Antimicrobial Resistance updated: local resistance rates removed, these were used to inform the guideline however are no longer stated in the guideline; importance of determining the nature of penicillin allergy highlighted.
- Chorioamnionitis, IAP, Pyrexia in Labour: Recommendation for vancomycin plus metronidazole plus gentamicin for patients with immediate-onset or severe penicillin hypersensitivity changed to clindamycin plus gentamicin empirically based on local GBS susceptibility data.
- Pyelonephritis: Recommendation for vancomycin plus gentamicin for patients with immediate-onset or severe penicillin hypersensitivity replaced with advice to contact Consultant Microbiologist for advice.
- C-section Wound Infection / Endometritis / Perineal Infection / Pelvic Infection post-ERPC / Infected Third or Fourth Degree Tear – Moderate to Severe : Recommendation for vancomycin plus metronidazole plus gentamicin for patients with immediate-onset or severe penicillin hypersensitivity changed to clindamycin plus gentamicin empirically based on local GBS susceptibility data.
- Meningococcal chemoprophylaxis section: Choice of agent removed, advice to refer to LH Guideline on Chemoprophylaxis for Meningococcal contacts 2018 added.
- LH VZV reference updated to current version.
Feb 2019
Shortage of pip/tazobactam - reserve for neutropenic sepsis and healthcare-associated pneumonia.
Alternative empiric treatment guidelines provided for acute cholangitis, healthcare-associated intra-abdominal infections, acute pancreatitis, diabetic foot ulcer deep infection or osteomyelitis, necrotising skin and soft tissue infections.
Nov 2018
Tile 3: Adult Treatment Guidelines
- Additional cautions on use of ciprofloxacin and levofloxacin added as per PRAC Review 2018 and CHMP final opinion.
- PO switch from cef-UR-oxime IV updated to Cefaclor for intra-abdominal infections and Cefalexin for urinary tract infections
- Encephalitis: Aciclovir dose in obesity changed from IBW to ODW based on updated literature review.
- Recommendations for SSIs clean-contaminated or dirty surgery updated.
Tile 9: Obstetrics and Gynaecology - updated that IAP should ideally be given 4 hours prior to delivery to optimise efficacy. Reference on indications for IAP updated to current OLOL Guideline.
Tile 12: Paediatric Guidelines
- Update of Quick Reference Paediatric Indication and Dose Poster.
- Addition of doses for cef-AZ-olin for all indications and for Azithromycin for pertussis as these are not available in the BNFc (References Sanford Guide 2018, OLCHC/TSCUH Hospital Antimicrobial Guidelines 2015). Removal of dose of aciclovir for encephalitis as this is available in the BNFc.
- Addition of caution each time that Ciprofloxacin is recommended based on CHMP Recommendations 2018.
- Removal of treatment regimen for peritonitis complicating peritoneal dialysis as not relevant to OLOL.
- Insertion of tallman lettering for cephalosporins throughout guideline to increase medication safety of these sound-alike-look-alike medications.
Oct 2018
Tile 12: Neonatal Guidelines
- Removal of section on microbiological investigations for suspected neonatal infections as this is now detailed in the OLOL Guideline for Prevention, Detection and Management of Neonatal Sepsis (Early and Late Onset), final draft 2018
- Removal of section on “targeted treatment of systemic infections caused by specific micro-organims” – not appropriate for empiric treatment guideline
- Neonatal antimicrobial prescribing principles: Removed advice to make a definite decision at 2 and 5 days of treatment.
- Neonatal Start Smart then Focus poster: Added “if no significant delay” to “take cultures before antimicrobials are started”, changed antibiotic review in “then Focus” section from 48 hours to 36 to 48 hours
- Candida – invasive infection: Added that duration should be discussed with Consultant Microbiologist
- Conjunctivitis: Duration of chloramphenicol changed to 48 hours after healing as per BNFc, treatment of severe conjunctivitis updated
- Meningitis – NICU Setting: Users advised to consult early-onset or late-onset neonatal sepsis guidelines as indicated
- NEC: Alternative regimen provided if prolonged therapy required or if renal impairment to avoid prolonged use of gentamicin
- Review of blood culture results updated from 48 hours to 36 hours incubation
- Late-onset neonatal sepsis: Treatment regimen updated from vancomycin plus gentamicin to flucloxacillin plus gentamicin unless the baby is MRSA colonised, in which case substitute vancomycin for flucloxacillin
- Septic arthritis and osteomyelitis: Suggested duration removed, discuss with Consultant Microbiologist
- Umbilical Infections: Added to check MRSA screen results
- UTI: Addition of caution to avoid prolonged duration of gentamicin - contact Consultant Microbiologist if alternative required
- UTI Prophylaxis: Added indication for prophylaxis
- Update of Primary Childhood Immunisation Schedule to current HSE schedule (October 2016)
- References updated.
Tile 12: Paediatric Guidelines
- Update of UTI recommendations based on update from Crumlin and Temple St Hospitals and review of local antimicrobial susceptibility patterns.
Oct 2018
Tile 1: Restricted Antibiotics - Ertapenem added as a restricted antibiotic in LH, gentamicin no longer restricted in LCH.
Tiles 3 and 5: Sepsis Community-Acquired: First Dose Antibiotics - national poster amended with local LH changes based on review of local antimicrobial resistance patterns
Tile 3: Adult treatment guidelines
- Hepatobiliary and pancreatic infections, intra-abdominal infections - change of co-amoxiclav to cef-UR-oxime and metronidazole based on review of local antimicrobial resistance patterns
- Urosepsis - change of co-amoxiclav and gentamicin to cef-UR-oxime and gentamicin based on review of local antimicrobial resistance patterns
Tile 4: Asplenia/splenic dysfunction - vaccination schedule updated as per updated National Immunisation Guidelines, July 2018.
Tile 6: Surgical Prophylaxis (Adults)
- Principles of surgical prophylaxis - removal of information on when to re-dose co-amoxiclav as co-amoxiclav no longer used for surgical prophylaxis
- Change of teicoplanin dose from 800mg set dose to 12mg/kg, rounded to the nearest 200mg
- Surgery performed in the setting of infection - change from surgical prophylaxis indicated in theatre to repeat dose of antimicrobials indicated if 4 hours have elapsed since the previous dose or if there is significant blood loss > 1,500ml (except do not re-dose gentamicin or teicoplanin/vancomycin, which have a prolonged action).
- Open fractures - addition that if MRSA cover required, teicoplanin should be added to the regimen.
Tile 7: Renal dosing
- All references updated
- Information for aciclovir oral and cef-AL-exin updated based on updated references.
July 2018
Tile 11: Gentamicin and vancomycin guidelines updated to include advice for patients on dialysis.
July 2018
Throughout:
-
References updated
-
Removal of references to OLH as now part of separate HSE Hospital Group
-
Summary of changes to previous versions moved from individual sections to Tile 1
Tile 1: Contact details updated
Tile 3: Sepsis Community-Acquired: First Dose Antibiotics - new section with ED Empiric Antibiotics for Sepsis Poster available at www.hse.ie (also located in Tile 5)
Tile 3: Bone and Joint Infections
-
Acute Osteomyelitis / Chronic Osteomyelitis / Septic Arthritis – Native Joint: Addition of key points to note as per Beaumont Hospital (BH). Removal of usual duration of treatment as dependent on many factors.
-
Acute Osteomyelitis / Septic Arthritis – Prosthetic Joint: Removal of advice to consider withholding treatment pending C&S if patient is medically stable.
-
Septic Arthritis – both sections: Addition of reminder that empiric rifampicin is not recommended.
Tile 3: Candidiasis – Mucocutaneous: New section
Tile 3: Infective Endocarditis
-
Infective Endocarditis – Community-Acquired or Late-Onset PVE: Empiric treatment changed from amoxicillin+flucloxacillin+gentamicin to vancomycin+gentamicin to include cover for community-acquired MRSA.
-
Infective Endocarditis: Usual duration of treatment removed as dependent on many factors.Additional Microbiological Ix as per BH.
Tile 3: Central Nervous System Infections - Meningitis:
-
Addition of request to consider dexamethasone.
-
Change of age to consider Listeria cover from 50 years to 65 years.
-
Update of penicillin allergy section to specify which regimen to use if patient pregnant
Tile 3: Gastrointestinal Infections
-
Acute gastro-enteritis: Added request for faeces sample for norovirus. Addition of “SIGHT” mnemonic as per BH.
-
Deletion of Clinical Management of CDI picture - relevant information included within treatment table for CDI.
-
H. pylori : Treatment choices and duration updates as per updated references.
Tile 3: Head and Neck Infections - Orbital Cellulitis: Empiric regimen updated based on review of references. Treatment duration changed from 10 to 14 days to 7 to 14 days.
Tile 3: Intra-abdominal Infections
-
Acute Pancreatitis: Addition of acute gallstone pancreatitis as an indication for antimicrobial treatment as per BH.
-
Community-Acquired and Healthcare-Associated Intra-Abdominal Infections: Addition of colonic perforation as an indication to consider antifungal treatment. Choice of antifungal no longer specified, advice to contact Consultant Microbiologist for choice.
-
Infected Pilonidal Sinus: New section.
Tile 3: Malaria - all sections updated based on updated references. Riamet® added as an option for oral stepdown for severe malaria and for P. vivax resistant to chloroquine.
Tile 3: Neutropenic Sepsis
- Now divided into two sections, initial empiric treatment and management of persistent fever despite empiric antimicrobials
- Increase of piperacillin/tazobactam dose from TDS to QDS as per BNF and SPC
- Advice to contact Consultant Microbiologist for advice if patient deteriorates clinically instead of automatic escalation to meropenem after 48 hours if no improvement
- Advice to discuss with Consultant Microbiologist if antifungal being considered, e.g. caspofungin instead of automatic addition of voriconazole if no improvement after 4 days.
Tile 3: Respiratory Tract Infections
-
Aspiration Pneumonia, Community Acquired: Removal of metronidazole if levofloxacin given for immediate onset or severe penicillin hypersensitivity.
-
Healthcare-Associated Pneumonia: Addition of vancomycin to piperacillin/tazobactam if history of MRSA.
-
IE Asthma / IE COPD: Clarified that if infiltrate on CXR, manage as for severe CAP.
-
Influenza treatment: New section.
-
Pleural Effusion with Pulmonary Infection / Suspected Empyema: New section.
Tile 3: Skin and Soft Tissue Infections
-
Cellulitis - Mild: Change of flucloxacillin PO dose from 500mg QDS to 500mg to 1g QDS.Addition that duration may be extended to 14 days for cellulitis in the setting of lymphoedema.
-
Infected superficial diabetic foot ulcer: Duration increased from 5 days to 7 days as per BH and IDSA Guidelines.
-
Diabetic Foot Ulcer: Infected deep ulcer – both sections.Update of advice to contact podiatry for review.
-
Diabetic Foot Ulcer: Infected deep ulcer with indications for hospitalisation – addition of comment to contact endocrinology team to facilitate rapid discharge if possible.
-
Diabetic Foot Osteomyelitis: New section.
-
Herpes zoster: New section.
Tile 3: Urinary Tract Infections
-
Asymptomatic bacteriuria: Comment updated.
-
Cystitis: Removal of information on local antimicrobial resistance.
-
Cystitis / UTI following TRUS Biopsy: Rewording of renal dosing for nitrofurantoin as per BNF.
-
Urosepsis / Pyelonephritis: Greater detail on duration of therapy.
-
Urosepsis post-TRUS prostate biopsy: Change of regimen from Meropenem+/-Gentamicin to Piperacillin/Tazobactam+Amikacin based on harmonisation with BH and local antimicrobial resistance rates.
Tile 3: Vascular Catheter Infections
-
PVC Infection: Rewording of duration of treatment for S. aureus BSI from 14 days to 14 days from date of first negative set of blood cultures.
-
CVC Infection: Added to contact Microbiology if antimicrobials indicated for CVC exit site infection in systemically well patient.
Tile 4: Adult Medical Prophylaxis
-
New section instead of tile for Administration of Antimicrobials.Administration of IV Antimicrobials Poster is on display on all wards in the IV prep area.
-
Asplenia: Update that first dose of vaccines may be given on discharge if patient is discharged sooner than 2 weeks post-splenectomy for practical reasons.
-
New sections on prophylaxis of influenza and variceal haemorrhage.
-
Meningococcal Prophylaxis for Contacts - replace current content with the phone number of Public Health and Occ Health and a link to the national guideline rather than copying information from the national guideline so that up to date information is always available.
Tile 5: Sepsis Management
-
Added ED Empiric Antibiotics for Sepsis Poster available at www.hse.ie (also located in Tile 3)
-
Removed HSE Sepsis Guide for Non-Pregnant Adults and images on Sepsis in Pregnant Patients, replaced with image of Sepsis 6 in Adults.
Tile 6: Adult Surgical Prophylaxis
-
Otolaryngology, Endocrine, Head and Neck Surgery Surgical Prophylaxis: Removed procedures not undertaken in OLOL.
-
TRUS Prostate Biopsy Prophylaxis: Update of prophylaxis from ciprofloxacin+amikacin for all patients to ciprofloxacin OR ciprofloxacin+amikacin OR discuss with Microbiology based on risk assessment.
-
Removal of surgical prophylaxis for percutaneous nephrolithotomy as procedure not undertaken in OLOL.
-
Removal of reconstitution information for Teicoplanin IV as this is available on the IV Administration Poster displayed in Theatre and on all wards.
Tile 7: Nitrofurantoin renal dosing updated as per BNF/SPC update.
Tile 8: OPAT - Remove information on cefazolin / probenecid dosing as it is only to be prescribed on OPAT if recommended by ID or Micro.
Tile 10: IV to Oral Switch Therapy – change of benzylpenicillin IV to PO switch option from phenoxymethylpenicillin to amoxicillin PO, addition of PO dose for clindamycin, levofloxacin and fluconazole, addition of cefuroxime IV to cefaclor LA 750mg BD PO.
Tile 11: Gentamicin and Vancomycin (Adults)
- Adult Gentamicin Guideline updated - specified to avoid duration in excess of 5 days, specified to check Ur/Cr daily or alternate days as indicated, changed trough level window from 18 to 24 hours to 16 to 24 hours, added Biochemistry level processing times, updated that daily levels required if renal impairment.
- Adult Vancomycin Guideline updated - round dose to the nearest 50mg, added that loading dose not recommended in pregnancy, added to adjust times of maintenance doses to 10am and 10pm to facilitate morning trough level checks, added Biochemistry level processing times, added comment that dose adjustment based on trough level result should also include a review of renal function and frequency of administration.
- Amikacin levels processed externally – replace comment with contact laboratory directly for result.
Tile 12: Paediatric Quick Reference Indication and Dose Poster added.
February 2018
Tile 7: Meropenem renal dosing updated.
Tile 6: Surgical prophylaxis for Caesarean section updated to cef-UR-oxime alone as per National Obs-Gynae Guidelines 2017.
Tile 9: Obstetrics and Gynaecology Guidelines updated:
- Chorioamnionitis: Change of regimen for patients with delayed-onset and non-severe penicillin hypersensitivity as per National Obs/Gynae Guidelines 2017 from cef-UR-oxime + gentamicin + metronidazole to cef-TRI-axone + gentamicin + metronidazole
- Update of PPROM Guideline to new regimen in Rotunda Hospital – based on Rotunda local antimicrobial resistance patterns, similar data not available in OLOL, follow Rotunda regimen as within our Hospital Group
- Severe Life-Threatening Ante-Natal Sepsis: Dose of clindamycin increased to 1.2g QDS IV as per National Obs/Gynae Guidelines. Gentamicin removed from regimen as meropenem considered to have sufficient gram negative cover.
- Change of PO cefalexin dose from 500mg QDS to 500mg TDS as per National Obs/Gynae Guidelines.
- Treatment of malaria in pregnancy updated based on updated references.
- Respiratory Tract Infections – Inpatient Treatment: Removal of gentamicin as part of regimen for patients with immediate-onset or severe penicillin hypersensitivity as per National Obs / Gynae Guidelines.
- Insertion of information on VZV post-exposure prophylaxis during pregnancy as per OLOL Guideline on Exposure to VZV during pregnancy
- Addition of section for treatment of vulvovaginal candidaisis as per National Obs / Gynae Guidelines.
- Pyrexia in Labour: Addition of benzylpenicillin 3g STAT dose to the regimen as per National Obs/Gynae Guidelines.
- Removal of guideline for confirmed iGAS infection as the guidelines are intended as empiric treatment guidelines. Confirmed iGAS is not included within the National Obs/Gynae Guidelines.
- Severe Mastitis/Breast Abscess: National Obs/Gynae Guidelines advise addition of clindamycin to flucloxacillin / cefuroxime / vancomycin. Decision by LH AMS Committee to add clindamycin to vancomycin due to concerns about subtherapeutic vancomycin levels initially. Clindamycin not considered necessary with flucloxacillin / cefuroxime based on local resistance patterns.
- Update of thrush guideline – topical miconazole for mother after each feed, not restricted to four times daily. Treatment duration changed from 1 to 2 weeks to continue until 7 days after symptoms have disappeared as per Rotunda Guideline.
- Severe Life-Threatening Post-Natal Sepsis: Dose of clindamycin increased to 1.2g QDS IV as per National Obs/Gynae Guidelines. Gentamicin removed from regimen as meropenem considered to have sufficient gram negative cover.
- New section added for sexually transmitted infections in pregnancy as per National Obs Gynae Guidelines.
- Update of Pelvic Inflammatory Disease Guideline based on updated references.
January 2018
Tile 12: Paediatric Guidelines added
Tile 1: Contact details updated
Tile 7: Oseltamivir renal dosing added
Dec 2017
Tile 12: Paediatric Gentamicin Guideline: Time that trough level can be taken increased to up to 8 hours before next dose due. Previously up to 6 hours before next dose due.
August 2016
Tile 1: Upate to contact details section.
July 2016
Tile 1: Updated contact information
Tile 1: Prescribing principles - added request that a senior member of team contact Consultant Microbiologist or ID Consultant for advice when possible.
Tile 1: Restricted antibiotics - meropenem now also restricted in OLH and LCH, gentamicin now also restricted in LCH.
Tile 3: Bone and Joint Infections
- Removal of recommendation that sodium fusidate may be added empirically for bone and joint infections due to high local MSSA resistance to fusidic acid in wound samples (20.7% in OLOL in 2015)
- Addition of cephalosporin-based alternative for patients with delayed-onset and non-severe penicillin hypersensitivity
- Osteomyelitis recommendations now divided into two sections, acute and chronic
- Update of empiric choice for prosthetic joint septic arthritis to vancomycin instead of flucloxacillin
- Update of rifampicin dose for prosthetic joint septic arthritis to 450mg BD (the higher end of recommended range as per IDSA Guidelines 2013) and advice that it should only be added for confirmed staphylococcal infection senstive to rifampicin.
Tile 3: Cardiovascular Infections
- Management of endocarditis updated as per ESC 2015 guidelines, including change of gentamicin to once daily dose
- Advise on catheter-related infections divided into two sections, PVC and CVC, and empiric recommendations updated.
Tile 3: Gastrointestinal infections - CDI section expanded with additional comments, no change to antimicrobial treatment options.
Tile 3: Genital Tract Infections
- Risk factors for STI-related acute epididmyo-orchitis updated as per BASHH guidelines.
- Recommendation for STI-related acute epididmyo-orchitis for patients with immediate-onset or severe penicillin hypersensitivity updated to recommend contacting ID Consultant for advice due to limited treatment options for N. gonorrhoeae .
Tile 3: Head and Neck Infections - empiric treatment of orbital cellulitis updated as per John Hopkins ABX Guide.
Tile 3: Hepatobiliary and Pancreatic Infections
- Addition of cephalosporin-based option where applicable for patients with delayed-onset and non-severe penicillin hypersensitivity
- Addition of advice to review gentamicin daily and to avoid durations in excess of 5 days
- Empiric treatment recommendation for acute pancreatitis changed based on updated literature review and harmonisation with practice in Beaumont Hospital
- Empiric treatment recommendation for spontaneous bacterial peritonitis changed based on updated literature review, local surveillance data and harmonisation with practice in Beaumont Hospital.
Tile 3: Intra-abdominal Infections
- Addition of cephalosporin-based alternative when applicable for patients with delayed-onset and non-severe penicillin hypersensitivity
- Addition of advice that gentamicin should be reviewed daily and to avoid durations in excess of 5 days.
- Update of advice regarding antifungal to name fluconazole as the empiric treatment option of choice if indicated.
Tile 3: Neutropenic Sepsis -updated empiric treatment recommendation for patients with penicillin allergy based on updated literature review and local surveillance data.
Tile 3: Respiratory Tract Infections
- NH-acquired pneumonia recategorised from healthcare-associated to community-acquired pneumonia as per NICE Guidelines 2014
- Section on community-acquired pneumonia expanded with additional comments.
- Addition of cephalosporin-based option when applicable for patients with delayed-onset and non-severe penicillin hypersensitivity.
- Section for hospital-acquired pneumonia removed, which advised to treat as either community-acquired or healthcare-associated based on time of onset - the healthcare-associated pneumonia section already specifies duration of > 5 days after admission.
- Empiric recommendation for community-acquired aspiration pneumonia for patients with penicillin allergy updated.
- Addition of sections for infective exacerbation of asthma and bronchietasis.
Tile 3: Skin and Soft Tissue Infections
- Removal of benzypenicillin / phenoxymethylpenicillin in combination with flucloxacillin for cellulitis based on antimicrobial spectrum of flucloxacillin and harmonisation with practice in Beaumont Hospital
- Addition of cephalosporin-based option where applicable for patients with delayed-onset and non-severe penicillin hypersensitivity
- Addition of section on toxic shock syndrome with advice to contact Consultant Microbiologist or ID Consultant for all suspected cases
- Update and expansion of empiric treatment recommendations for diabetic foot ulcers based on literature review, local surveillance data and harmonisation with practice in Beaumont Hospital
- Change of penicillin allergy alternative for skin and soft tissue infections from clarithromycin to doxycycline based on harmonisation with practice in Beaumont Hospital
- Change of penicillin allergy alternative for cellulitis from vancomycin to clindamycin based on harmonisation with practice in Beaumont Hospital
- Change of empiric treatment recommendation for human or animal bites for patients with penicillin allergy to doxycycline and metronidazole based on harmonisation with practice in Beaumont Hospital.
Tile 3: Urinary Tract Infections
- Addition of section for asymptomatic bacteriuria
- Addition of advice for UTI as well as urosepsis post-TRUS prostate biopsy
- Addition of advice to review gentamicin daily and avoid duration in excess of 5 days
- Change of empiric treatment option for urosepsis post-TRUS prostate biopsy from meropenem +/- amikacin to meropenem +/- gentamicin based on local surveillance data.
Tile 4: Administration of aztreonam added, administration of pip/tazobactam updated, removal of divided dose gentamicin administration advice.
Tile 6: Adult Surgical Prophylaxis - main changes include:
- Updated dose of teicoplanin to 800mg stat IV for orthopaedic procedures
- Update of surgical prophylaxis regimen for open fractures
- Updated obs-gynae surgical prophylaxis section with two alternatives for penicillin allergy, depending on the nature of the allergy
- Update of surgical prophylaxis for TURB-T procedures to advice that antimicrobial prophylaxis is not indicated.
Tile 7: Renal dose for aztreonam added, renal dose of gentamicin updated as per updated LH and OLH Gentamicin Once Daily Guideline 2016.
Tile 8: Addition of local practice information regarding treatment of cellulitis on OPAT.
Tile 10: Update of oral dose amoxicillin and flucloxacillin.
Tile 11: Update of adult gentamicin guideline to include new renal dose and to remove section on divided dose gentamicin as this is no longer indicated.
Tile 12: Neonatal antimicrobial monographs for aciclovir and fluconazole added.
December 2015
Tile 3: Meningitis guideline updated.
Tile 3: Vancomycin dosing updated to the new dosing recommendation in each individual section.
Tile 3: Meningitis Guideline updated to include cephalosporin-based option for patients with delayed-onset and non-severe penicillin allergy and to recommend meropenem (with caution and monitoring) for patients with immediate-onset or severe penicillin hypersensitivity as per HPSC Guidelines 2012.
November 2015
Tile 11: Vancomycin guideline updated with Version 2, Nov 2015 - loading dose now recommended for all patients.
Tile 12: Paediatric Gentamicin guideline updated - first trough level now due pre-3rd dose if normal renal function and pre-2nd dose if renal impairment. Previously pre-2nd dose for all patients.
August 2015
Tile 12: Neonatal antimicrobial monographs added for Ambisome, Gentamicin and Metronidazole (first version).
July 2015
Tile 1: Updated contact information for Consultant Microbiologists
Tile 6: Principles of surgical antibiotic prophylaxis updated.
Tile 6: Abdominal, Gastrointestinal and General Surgery Antibiotic Prophylaxis updated.
Tile 12: Renamed "Neonatal and Paediatric Guidelines".
Tile 12: Neonatal monograph for benzATHINE penicillin IM added (first version).
Tile 12: Paediatric Gentamicin once daily guideline added (first version).
May 2015
Tile 1: Prescribing principles updated - added advice to check safety of antimicrobial if patient is pregnant or breast-feeding, added reminder to review empiric therapy after 48 hours in conjunction with C&S results.
Tile 2: Penicillin hypersensitivity updated that meropenem may be used with great caution and close clinical monitoring if history of immediate-onset or severe hypersensitivity reactions to penicillin. In the antimicrobial treatment guidelines, meropenem has been recommended in very limited circumstances in this patient group with advice on caution and monitoring as above. Also, the section was updated to advise that it is crucial to diffferentiate between immediate-onset and delayed-onset penicillin hypersensitivity reactions.
Tile 3: Pelvic Inflammatory Disease updated as per Obs-Gynae Guidelines.
Tile 5: Sepsis Management - references to NCEC Sepsis Management Guideline and OLOL clinical guideline on the management of maternal sepsis added, images for management of sepsis in pregnant patients added.
Tile 6: Obstetrics and Gynaecology surgical prophylaxis added (first version).
Tile 9: Obstetrics and Gynaecology antimicrobial guidelines added (first version).
Tile 12: Rainbow guidelines reference updated to 2015 edition.
February 2015
Tile 12: Neonatal antimicrobial guidelines added (first version).
Version 1.0 November 2014
This is the first version of the smartphone application guideline.

