Surgical Prophylaxis
Principles of Surgical Prophylaxis
Goal of surgical prophylaxis
The goal of surgical antibiotic prophylaxis is to prevent surgical site infection. The choice of agent(s) is determined by the likely potential pathogens at the operative site.
Choice of surgical prophylaxis
- The choice of agent will be governed by the procedure and the likely potential pathogen.
- The choice of agent may also be influenced by recent or previous infection, prolonged hospital stay, or colonisation with MRSA or other resistant organisms. In such circumstances, it is advisable to consult the latest culture and sensitivity reports and consult with Microbiology.
- An agent that may be appropriate for surgical prophylaxis may not be the optimal agent for the therapy of established infection.
- All active infections should be under treatment prior to surgery.
- Patients should have their tetanus status checked if a contaminated wound is present, as well as receiving prophylactic antimicrobials. Please refer to the Immunisation Guidelines for Ireland for latest guidance on risk assessment.
Timing of surgical prophylaxis
Surgical antibiotic prophylaxis should be fully administered within 60 minutes before the first incision to ensure maximum blood and tissue levels at the time of first incision. In surgery where a tourniquet is to be applied, a 15 minute period is required between the end of prophylactic antibiotic administration and tourniquet application.
Number of doses of surgical prophylaxis
A single pre-operative dose is recommended for surgical prophylaxis except for orthopaedic procedures where up to 24 hours prophylaxis may be indicated.
A second dose intra-operatively is indicated if:
(1) the duration of the operation is longer than 4 hours
(2) if there is significant blood loss of more than 1,500mL for adults or 25mL/kg for paediatrics. In this case , the second dose of antibiotic should be given after fluid replacement.
- A second dose of gentamicin and teicoplanin/vancomycin is NOT indicated due to prolonged action
- The redosing interval should be measured from the time of administration of the pre-operative dose, not from the beginning of the procedure.
Dose of each agent
|
ANTIBIOTIC |
ADULT DOSE |
PAEDIATRIC DOSE |
|
Cef-UR-oxime |
1.5g IV bolus |
50mg/kg IV bolus (max 1.5g) |
|
Metronidazole |
500mg IV infusion over 20 mins |
15mg/kg IV infusion over 20 mins (max 500mg) |
|
Gentamicin |
5mg/kg IV bolus (max 480mg) Renal Dose: 3mg/kg IV bolus Obesity: If BMI > 30kg/m 2 , use gentamicin calculator to calculate obesity-adjusted dose |
7mg/kg IV bolus (max 480mg) Renal Dose: 2 – 5 mg/kg IV bolus Obesity: If BMI > 30kg/m 2 , use ideal body weight to calculate dose |
|
Teicoplanin |
12mg/kg IV bolus (rounded to the nearest 200mg) |
Child 1 – 2 months: 16mg/kg IV bolus Child > 2 months: 10mg/kg IV bolus |
|
Clindamycin |
900mg IV infusion over 30 mins |
- |
MRSA considerations
- All orthopaedic patients should be screened for MRSA as per LH Infection Prevention and Control Guidelines.
- MRSA Eradication Protocol: Intranasal mupirocin three times daily for 5 days PLUS chlorhexidine body washes.
- The MRSA eradication protocol should be used prophylactically for adult patients undergoing surgery with a high risk of major morbidity who are identified with S. aureus or MRSA.
- For MRSA positive patients, teicoplanin should be administered for surgical prophylaxis.
- Teicoplanin should also be considered in cases where MRSA is specifically suspected, for example, patients with a known history of MRSA colonisation without documented eradication and patients at high risk for MRSA colonisation in the absence of surveillance data, eg. patients who were recently hospitalised, transferred from another healthcare institution or nursing home residents.
Caution - risk of anaphylaxis with teicoplanin
-
Anaphylactic reaction / anaphylaxis occurs uncommonly ( > 1/1,000 to <1/100) as per Summary of Product Characteristics
-
NAP6 Report 2018:
-
Overall incidence of peri-operative anaphylaxis due to antibiotics was estimated at 4 per 100,000 exposures.
-
The highest estimated incidence was for teicoplanin (16.4 per 100,000 exposures) followed by co-amoxiclav (8.7 per 100,000 exposures).Over half of patients who reacted to teicoplanin reported pre-operatively that they were allergic to penicillin.
-
Hypotension was the most common presenting feature.
-
Onset of symptoms was less than 10 minutes in 92% of cases and less than 30 minutes in all cases, therefore administration several minutes before induction of anaesthesia would likely improve detection and may simplify treatment.
-
Definition of Surgical Site Infection
CDC/NHSN Surveillance Definition of Surgical Site Infection:
See https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf for full information.
Superficial Incisional :
Date of event occurs within 30 days following the NHSN operative procedure (where day 1 = the procedure date) AND involves only skin and subcutaneous tissue of the incision AND patient has at least one of the following:
a. purulent drainage from the superficial incision.
b. organism(s) identified from an aseptically-obtained specimen from the superficial incision or subcutaneous tissue by a culture or nonculture based microbiologic testing method which is performed for purposes of clinical diagnosis or treatment.
c. a superficial incision that is deliberately opened by a surgeon, physician or physician designee and culture or non-culture based testing of the superficial incision or subcutaneous tissue is not performed
AND
patient has at least one of the following signs or symptoms: localized pain or tenderness; localized swelling; erythema; or heat.
d. diagnosis of a superficial incisional SSI by a physician or physician designee.
There are two specific types of superficial incisional SSIs:
1. Superficial Incisional Primary (SIP) – a superficial incisional SSI that is identified in the primary incision in a patient that has had an operation with one or more incisions (for example, C-section incision or chest incision for CBGB).
2. Superficial Incisional Secondary (SIS) – a superficial incisional SSI that is identified in the secondary incision in a patient that has had an operation with more than one incision (for example, donor site incision for CBGB).
Deep Incisional :
Date of event occurs within 30 or 90 days following the NHSN operative procedure (where day 1 = the procedure date) according to the list in Table 2 AND involves deep soft tissues of the incision (for example, fascial and muscle layers) AND patient has at least one of the following:
a. purulent drainage from the deep incision.
b. a deep incision that is deliberately opened or aspirated by a surgeon, physician or physician designee or spontaneously dehisces
AND
organism(s) identified from the deep soft tissues of the incision by a culture or non-culture based microbiologic testing method which is performed for purposes of clinical diagnosis or treatment or culture or nonculture based microbiologic testing method is not performed. A culture or non-culture based test from the deep soft tissues of the incision that has a negative finding does not meet this criterion
AND
patient has at least one of the following signs or symptoms: fever (>38°C); localized pain or tenderness.
c. an abscess or other evidence of infection involving the deep incision detected on gross anatomical exam, histopathologic exam, or imaging test.
There are two specific types of deep incisional SSIs:
1. Deep Incisional Primary (DIP) – a deep incisional SSI that is identified in a primary incision in a patient that has had an operation with one or more incisions (for example, C-section incision or chest incision for CBGB).
2. Deep Incisional Secondary (DIS) – a deep incisional SSI that is identified in the secondary incision in a patient that has had an operation with more than one incision (for example, donor site incision for CBGB).
Organ/Space :
Date of event occurs within 30 or 90 days following the NHSN operative procedure (where day 1 = the procedure date) according to the list in Table 2 AND involves any part of the body deeper than the fascial/muscle layers that is opened or manipulated during the operative procedure AND patient has at least one of the following:
a. purulent drainage from a drain placed into the organ/space (for example, closed suction drainage system, open drain, T-tube drain, CTguided drainage).
b. organism(s) identified from fluid or tissue in the organ/space by a culture or non-culture based microbiologic testing method which is performed for purposes of clinical diagnosis or treatment.
c. an abscess or other evidence of infection involving the organ/space detected on gross anatomical exam or histopathologic exam, or imaging test evidence definitive or equivocal for infection.
AND
meets at least one criterion for a specific organ/space infection site listed in Table 3.
RCPI Surgical Site Infection Prevention Care Bundle (2012)
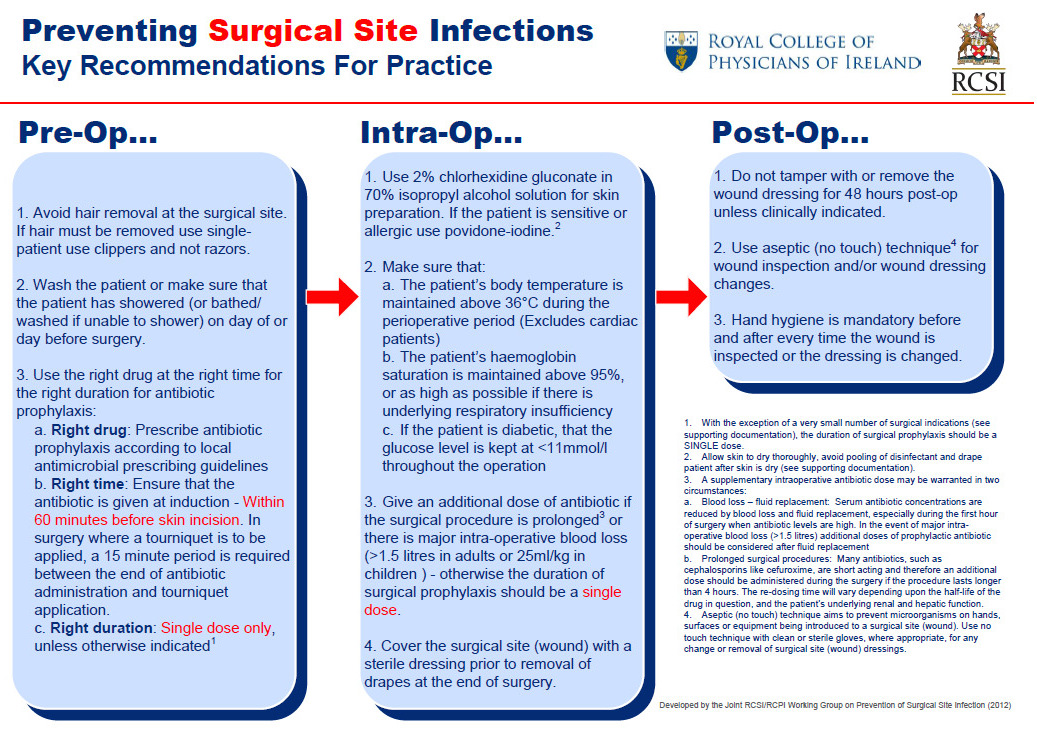
Reproduced with permission from RCPI/RCSI Working Group on Prevention of Surgical Site Infection
Adult Surgical Prophylaxis
Abdominal, Gastrointestinal and General Surgery Prophylaxis
|
Procedure |
|
|
Gastrointestinal and Hepatobiliary
|
|
|
First Line Antimicrobials |
|
|
Cef-UR-oxime 1.5g IV bolus AND Metronidazole 500mg IV infusion If MRSA cover required , add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg |
|
|
Penicillin Allergy Alternative |
|
|
DELAYED-onset Penicillin Hypersensitivity Cef-UR-oxime 1.5g IV bolus AND Metronidazole 500mg IV infusion If MRSA cover required , add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg AND Gentamicin 5mg/kg IV bolus (renal dose 3mg/kg IV). AND Metronidazole 500mg IV infusion |
|
|
Procedure |
|
Hernia repair with and without mesh insertion |
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g IV bolus If MRSA cover required , add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg |
|
Penicillin Allergy Alternative |
|
DELAYED-onset Penicillin Hypersensitivity Cef-UR-oxime 1.5g IV bolus If MRSA cover required , add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg AND Gentamicin 5mg/kg IV bolus (renal dose 3mg/kg IV). |
|
Procedure |
|
Percutaneous Endoscopic Gastrostomy (PEG) insertion |
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g IV bolus If MRSA cover required , add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg |
|
Penicillin Allergy Alternative |
|
DELAYED-onset Penicillin Hypersensitivity Cef-UR-oxime 1.5g IV bolus If MRSA cover required , add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg AND Gentamicin 5mg/kg IV bolus (renal dose 3mg/kg IV). |
|
Procedure |
|
Elective splenectomy |
|
First Line Antimicrobials |
|
Antibiotic prophylaxis is not recommended, however may be considered in high risk patients, eg. immunosuppressed patients. |
|
Procedure |
|
Appendicectomy |
|
First Line Antimicrobials |
|
|
Procedure |
|
Laparoscopy or Laparotomy for Peritonitis, Perforation, Abscess Drainage |
|
First Line Antimicrobials |
|
Otolaryngology, endocrine, head & neck surgery
|
Procedure |
|
Thyroid Surgery |
|
Comment |
|
Antibiotic prophylaxis is not indicated unless neck dissection performed. |
|
Procedure |
|
Parathyroid Surgery |
|
Comment |
|
Antibiotic prophylaxis is not indicated unless neck dissection performed. |
|
Procedure |
|
Neck Lymph Node Excision |
|
Comment |
|
Antimicrobial prophylaxis is not indicated. Always consider possibility of mycobacterial infection and send specimens to both histopathology (in formalin) and microbiology (in normal saline). |
|
Procedure |
|
Tonsillectomy |
|
Comment |
|
Antimicrobial prophylaxis is not indicated. Antimicrobial therapy indicated if active infection/abscess present (Refer to peritonsillar abscess treatment guideline). |
|
Procedure |
|
Adenoidectomy |
|
Comment |
|
Antimicrobial prophylaxis is not indicated. Antimicrobial therapy indicated if active infection/abscess present (Refer to peritonsillar abscess treatment guideline). |
|
Procedure |
|
Grommet Insertion |
|
First Line Antimicrobials |
|
CILOXAN® (ciprofloxacin) Adult: 4 drops into affected ear – single dose intra-operatively Child: 3 drops into affected ear - single dose intra-operatively |
|
Procedure |
|
Clean Ear Surgery |
|
Comment |
|
Antimicrobial prophylaxis is not indicated. |
|
Procedure |
|
Clean-Contaminated Ear Surgery |
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g IV bolus If MRSA cover required, add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg |
|
Penicillin Allergy Alternative |
|
DELAYED-onset Penicillin Hypersensitivity Cef-UR-oxime 1.5g IV bolus If MRSA cover required, add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg AND Gentamicin 5mg/kg IV bolus (renal dose 3mg/kg IV) |
|
Comment |
|
Check whether the patient is already known to be colonised with MRSA. Screen pre-operatively for MRSA carriage (nose, throat, groin and other sites, as appropriate). If MRSA is detected, prescribe MRSA decolonisation protocol pre-operatively. |
|
Procedure |
|
Functional Endoscopic Sinus Surgery (FESS) |
|
Comment |
|
Antimicrobial prophylaxis is generally not indicated Antimicrobial therapy indicated if FESS performed for chronic rhinosinusitis OR infection/abscess present (Refer to chronic rhinosinusitis treatment guideline). |
|
Procedure |
|
Complex Septo-Rhinoplasty |
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g IV bolus AND Metronidazole 500mg IV infusion If MRSA cover required, add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg |
|
Penicillin Allergy Alternative |
|
DELAYED-onset Penicillin Hypersensitivity Cef-UR-oxime 1.5g IV bolus AND Metronidazole 500mg IV infusion If MRSA cover required, add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg AND Gentamicin 5mg/kg IV bolus (renal dose 3mg/kg IV) AND Metronidazole 500mg IV infusion |
Trauma and Orthopaedic Surgery Prophylaxis
|
Procedure |
|
Arthroplasty including Hip Fracture Repair and Total Joint Replacement N.B. Please see also MRSA Considerations :
|
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g IV bolus If MRSA cover required, add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg Consider up to 24 hours of prophylaxis with Cef-UR-oxime 1.5g TDS IV. Teicoplanin single dose is sufficient for 24 hours of prophylaxis. |
|
Penicillin Allergy Alternative |
|
DELAYED-onset Penicillin Hypersensitivity Cef-UR-oxime 1.5g IV bolus If MRSA cover required, add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg Consider up to 24 hours of prophylaxis with Cef-UR-oxime 1.5g TDS IV. Teicoplanin single dose is sufficient for 24 hours of prophylaxis. IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg Teicoplanin single dose is sufficient for 24 hours of prophylaxis. |
|
Procedure |
|
Open Fractures
N.B. Please see also MRSA Considerations :
|
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g IV bolus AND Metronidazole 500mg IV infusion If MRSA cover required, add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg. If patient has already received vancomycin on the ward and next dose not yet due at time of surgery, omit teicoplanin. Vancomycin recommended if treatment to continue post-operatively. |
|
Penicillin Allergy Alternative |
|
DELAYED-onset and non-severe Penicillin Hypersensitivity Cef-UR-oxime 1.5g IV bolus AND Metronidazole 500mg IV infusion If MRSA cover required, add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg. If patient has already received vancomycin on the ward and next dose not yet due at time of surgery, omit teicoplanin. Vancomycin recommended if treatment to continue post-operatively. IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg. If patient has already received vancomycin on the ward and next dose not yet due at time of surgery, omit teicoplanin. Vancomycin recommended if treatment to continue post-operatively. AND Metronidazole 500mg IV infusion AND ONLY IF type III fracture with extensive soft tissue injury – STAT dose in Theatre for surgical prophylaxis (i.e. not to be continued as part of treatment regimen): Gentamicin 5mg/kg IV (renal dose 3mg/kg IV). |
|
Procedure |
|
ORIF Procedures (e.g. Nails, Screws, Plates, Wires) N.B. Please see also MRSA Considerations :
|
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g IV bolus If MRSA cover required, add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg Consider up to 24 hours of prophylaxis with Cef-UR-oxime 1.5g TDS IV. Teicoplanin single dose is sufficient for 24 hours of prophylaxis. |
|
Penicillin Allergy Alternative |
|
DELAYED-onset Penicillin Hypersensitivity Cef-UR-oxime 1.5g IV bolus If MRSA cover required, add Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg Consider up to 24 hours of prophylaxis with Cef-UR-oxime 1.5g TDS IV. Teicoplanin single dose is sufficient for 24 hours of prophylaxis. IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity Teicoplanin 12mg/kg IV bolus, rounded to the nearest 200mg A single dose is sufficient for 24 hours of prophylaxis. |
Urogenital Surgery Prophylaxis
|
Procedure |
|
|
First Line Antimicrobials |
|
Antimicrobial prophylaxis is not generally indicated. If the patient is at high risk of endocarditis and/or is immunocompromised, send urine for C&S within 5 days prior to procedure. Actions:
Contact Consultant Microbiologist to discuss further if necessary. |
|
Procedure |
|
Endoscopic Ureteric Stone Fragmentation |
|
First Line Antimicrobials |
|
Gentamicin 5mg/kg IV ( renal dose 3mg/kg IV). |
|
Procedure |
|
Extracorporeal Shock Wave Lithotripsy |
|
First Line Antimicrobials |
|
Gentamicin 5mg/kg IV ( renal dose 3mg/kg IV). |
|
Procedure |
|
Trans Rectal Ultrasound (TRUS) Guided Prostate Biopsy |
|
First Line Antimicrobials |
|
N.B. Risk Assessment Questionnaire to be completed for all patients by the referring Urologist to determine appropriate prophylaxis. If healthcare worker / quinolone use in the last 6 months : Ciprofloxacin 750mg PO one hour prior to procedure and 750mg PO 12 hours after procedure (supply single dose to patient on discharge) AND Amikacin 15mg/kg IM STAT (max 1g), one hour prior to procedure (use 500mg/2ml vials) If antimicrobial treatment for UTI in the last 12 months :
If history of infection following a previous TRUS prostate biopsy : Choice of prophylaxis to be discussed with Consultant Microbiologist If history of CRE/CPE or if patient has risk factors for CRE/CPE : Rectal swab for CPE/CRE carriage required prior to biopsy. Result should be reviewed before biopsy proceeds. Contact Consultant Microbiologist for further advice if required. All other patients : Ciprofloxacin 750mg PO one hour prior to procedure and 750mg PO 12 hours after procedure (supply single dose to patient on discharge) |
|
Procedure |
|
Transurethral Resection of Bladder Tumour (TURBT) |
|
First Line Antimicrobials |
|
Gentamicin 5mg/kg IV ( renal dose 3mg/kg IV). |
|
Procedure |
|
Transurethral Resection of the Prostate (TURP) |
|
First Line Antimicrobials |
|
Gentamicin 5mg/kg IV ( renal dose 3mg/kg IV). |
|
Procedure |
|
Ureteric Stenting |
|
First Line Antimicrobials |
|
Gentamicin 5mg/kg IV (renal dose 3mg/kg IV). |
Obstetrics and Gynaecology Surgical Prophylaxis
|
Procedure |
|
Caesarean Section |
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g IV If MRSA cover required, add Teicoplanin 800mg IV |
|
NON-IMMEDIATE-onset Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g IV If MRSA cover required, add Teicoplanin 800mg IV bolus |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Clindamycin 900mg IV AND Gentamicin 5mg/kg IV ( renal dose 3mg/kg IV) If MRSA cover required, add Teicoplanin 800mg IV bolus |
|
Procedure |
|
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g IV AND Metronidazole 500mg IV If MRSA cover required, add Teicoplanin 800mg IV |
|
NON-IMMEDIATE-onset Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g IV AND Metronidazole 500mg IV If MRSA cover required, add Teicoplanin 800mg IV bolus |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Clindamycin 900mg IV AND Gentamicin 5mg/kg IV ( renal dose 3mg/kg IV) If MRSA cover required, add Teicoplanin 800mg IV bolus |
|
Procedure |
|
Assisted/ operative vaginal delivery (forceps or vacuum) Give single-dose prophylaxis as soon as possible after delivery (Ref. ANODE trial Lancet 2019) See also Operative Vaginal Deliveries guideline (OLOLH-DEP-OBS-162) October 2020, available on Qpulse |
|
First Line Antimicrobials |
|
Co-amoxiclav 1.2g IV |
|
NON-IMMEDIATE-onset Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g IV |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Clindamycin 900mg IV |
|
Procedure |
|
|
First Line Antimicrobials |
|
Cef-UR-oxime 1.5g IV If MRSA cover required, add Teicoplanin 800mg IV |
|
NON-IMMEDIATE-onset Penicillin Hypersensitivity |
|
Cef-UR-oxime 1.5g IV If MRSA cover required, add Teicoplanin 800mg IV bolus |
|
IMMEDIATE-onset or SEVERE Penicillin Hypersensitivity |
|
Clindamycin 900mg IV AND Gentamicin 5mg/kg IV ( renal dose 3mg/kg IV) If MRSA cover required, add Teicoplanin 800mg IV bolus |
|
Procedure |
|
|
First Line Antimicrobials |
|
Surgical Antibiotic Prophylaxis not recommended |
|
Procedure |
|
|
First Line Antimicrobials |
If procedure demonstrates dilated fallopian tubes, treatment course of doxycycline 100mg BD for 7 days is recommended. |
|
Procedure |
|
|
First Line Antimicrobials |
|
Please note that testing for chlamydia is usually undertaken as part of workup prior to these investigations:
Where testing for chlamydia has not been carried out:
If procedure demonstrates dilated fallopian tubes, treatment course of doxycycline 100mg BD for 7 days is recommended |
|
Procedure |
|
Ureteric Stenting |
|
First Line Antimicrobials |
Gentamicin 5mg/kg IV ( renal dose 3mg/kg IV) |
Paediatric Surgical Prophylaxis
Paediatrics - General Surgery Prophylaxis
|
Procedure |
|
Paediatrics - Appendicectomy: Appendix Normal or Inflamed |
|
Prophylactic Antimicrobials |
|
First Line Prophylaxis/ Delayed-Onset Penicillin Hypersensitivity: Cef-UR-oxime IV AND Metronidazole IV If MRSA cover required, Add Teicoplanin IV Immediate-Onset or Severe Penicillin Hypersensitivity: Teicoplanin IV AND Gentamicin IV AND Metronidazole IV |
|
Duration of Prophylaxis |
|
Uncomplicated appendicitis: No further post op doses necessary |
|
Comments |
|
If patient chronically colonised with bacteria other than MRSA, contact Microbiology for advice. |
|
Procedure |
|
Paediatrics - Appendicectomy: Appendix Gangrenous or Perforated |
|
Prophylactic Antimicrobials |
|
First Line Prophylaxis/ Delayed-Onset Penicillin Hypersensitivity: Cef-UR-oxime IV AND Metronidazole IV AND Gentamicin IV If MRSA cover required, Add Teicoplanin IV Immediate-Onset or Severe Penicillin Hypersensitivity: Teicoplanin IV AND Gentamicin IV AND Metronidazole IV |
|
Duration |
|
Treatment course required post-op: Continue for 5 days (switch IV to PO when clinically appropriate). |
|
Comments |
|
If patient chronically colonised with bacteria other than MRSA, contact Microbiology for advice. |
|
Procedure |
|
Paediatrics - Insertion of Non-tunnelled and Tunnelled Central Lines |
|
Prophylactic Antimicrobials |
|
Antimicrobial prophylaxis not recommended. |
Paediatrics - Endocarditis Prophylaxis for Dental Procedures
|
Paediatrics - Endocarditis Prophylaxis for Dental Procedures |
|
Required for: Dental procedures with manipulation of gingival or apical region of teeth, or perforation of oral mucosa. Not required for:
|
|
Underlying Cardiac Condition |
|
Prophylaxis only required if:
|
|
Prophylactic Antimicrobials |
|
First Line: Amoxicillin 50mg/kg PO or IV (max 2g) (single dose, 30-60 minutes before procedure) Penicillin Hypersensitivity: Clindamycin 20mg/kg PO or IV (max 600mg) (single dose, 30-60 minutes before procedure) Reference: Health Service Executive. Antibiotic Prescribing; Dental; Endocarditis Prophylaxis. Last updated April 2019. Available from www.hse.ie |
Paediatrics - ENT Surgery Prophylaxis
|
Procedure |
|
Paediatrics - Adenoidectomy, Insertion of Grommets, Tonsillectomy |
|
Prophylactic Antimicrobials |
|
Antibiotics not usually required unless infection or abscess present. |
|
Procedure |
|
Paediatrics - Mastoidectomy, Tympanoplasty, Ear Surgery e.g. Pinnoplasty, Nose or Sinus Surgery |
|
Prophylactic Antimicrobials |
|
First Line Prophylaxis/ Delayed-Onset Penicillin Hypersensitivity: Cef-UR-oxime IV If MRSA cover required, Add Teicoplanin IV Immediate-Onset or Severe Penicillin Hypersensitivity: Teicoplanin IV AND Gentamicin IV |
|
Duration of Prophylaxis |
|
No further post op doses necessary |
Paediatrics - Orthopaedic Surgery Prophylaxis
|
Procedure |
|
Paediatrics - Closed Clean Procedures without Prosthesis/Implants |
|
Prophylactic Antimicrobials |
|
Antimicrobial prophylaxis not recommended. |
|
Procedure |
|
Paediatrics - Standard Procedures with Prosthesis/Implants |
|
Prophylactic Antimicrobials |
|
First Line Prophylaxis/ Delayed-Onset Penicillin Hypersensitivity: Cef-UR-oxime IV If MRSA cover required, Add Teicoplanin IV Immediate-Onset or Severe Penicillin Hypersensitivity: Teicoplanin IV |
|
Duration of Prophylaxis |
|
No further post op doses necessary |
|
Comments |
|
If patient chronically colonised with bacteria other than MRSA, contact Microbiology for advice. |
|
Procedure |
|
Paediatrics - Open Fractures / Wounds N.B . Intravenous antibiotics are administered as soon as possible after injury and continued until wound debridement. Administer antibiotic doses pre-operatively (no more than 60 minutes before skin incision) to ensure optimum plasma and tissue concentrations at the time of procedure. |
|
Prophylactic Antimicrobials |
|
First Line Prophylaxis/ Delayed-Onset Penicillin Hypersensitivity: Cef-UR-oxime IV AND Metronidazole IV If MRSA cover required, Add Teicoplanin IV Immediate-Onset or Severe Penicillin Hypersensitivity: Teicoplanin IV AND Gentamicin IV AND Metronidazole IV |
|
Duration |
|
Continue for 72 hours or until definitive wound closure whichever is sooner. |
|
Comments |
|
If patient chronically colonised with bacteria other than MRSA, contact Microbiology for advice. |
Paediatrics - Plastic Surgery Prophylaxis
|
Procedure |
|
Paediatrics - Trauma Wounds (e.g. Nail Bed Injury) |
|
Prophylactic Antimicrobials |
|
First Line Prophylaxis/ Delayed-Onset Penicillin Hypersensitivity: Cef-UR-oxime IV AND Metronidazole IV If MRSA cover required, Add Teicoplanin IV Immediate-Onset or Severe Penicillin Hypersensitivity: Teicoplanin IV AND Gentamicin IV AND Metronidazole IV |
|
Duration of Prophylaxis |
|
No further post op doses necessary |
|
Comments |
|
If patient chronically colonised with bacteria other than MRSA, contact Microbiology for advice. |
References
-
Beaumont Hospital Antimicrobial Guidelines 2018. Available from RCSI Hospitals Antimicrobial Guidelines Smartphone Application, accessed 12/03/18.Obtained with permission from Consultant Microbiologist.
-
National Cancer Control Programme National Prostate Biopsy Infection Project Board. National policy on the prevention and management of infection post transrectal ultrasound (TRUS) guided prostate biopsy, 2014. Available from www.hse.ie .
-
RCPI/RCSI Working Group on Prevention of Surgical Site Infections. Preventing Surgical Site Infections: Key recommendations for practice, 2012. Available from www.rcpi.ie .
-
Scottish Intercollegiate Guidelines Network. SIGN 104: Antibiotic prophylaxis in surgery. 2014. Available from www.sign.ac.uk .
-
Bratzler DW, Dellinger ED, Olsen KM et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013;70:195-283.
-
Wockhardt UK Limited.Co-amoxiclav 1000mg/200mg Summary of Product Characteristics, 2015. Available from www.hpra.ie , accessed 9/5/16.
-
American Society for Gastrointestinal Endoscopy. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2008;67(6):791-6.
-
Allison MC, Sandoe JAT, Tighe R et al. Antibiotic prophylaxis in gastrointestinal endoscopy. British Society of Gastroenterology. Gut 2009;58:869–880.
-
Hoff WS, Bonadies JA, Cachecho R, et al. East Practice Management Guidelines Work Group: Update to Practice Management Guidelines for Prophylactic Antibiotic Use in Open Fractures. J Trauma 2011;70(3):751-754.
-
A Strategy for the Control of Antimicrobial Resistance in Ireland (SARI). Guidelines for the prevention of catheter-associated urinary tract infection. 2011. Available from www.hpsc.ie .
-
Wolf JS, Bennett CJ, Dmochowski RR et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. American Urological Association, 2014. Available from www.auanet.org .
-
Health Products Regulatory Authority. Summary of Product Characteristics for Targocid®, available from www.hpra.ie , accessed 11.03.19.
-
Cook T, Harper N [Editors]. Anaesthesia, Surgery and Life-Threatening Allergic Reactions. Report and Findings of the Royal College of Anaesthetists’ 6 th National Audit Project: Perioperative anaphylaxis. RCoA, HSRC and NAP; May 2018. Available from www.nationalauditprojects.org.uk .
- Soper DE, Chelmow D, ACOG Committee on Practice Bulletins – Gynecology. ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures. Obstetrics & Gynecology 2018; 131(6):e172-e189.
- Eyk NV and van Schalkwyk J. Society of Obstetricians and Gynaecologists of Canada Clinical Practice Guideline. Antibiotic prophylaxis in gynaecologic procedures. No. 275, April 2012. Available from sogc.org , accessed 25/03/15.
- CDC/NHSN Surgical Site Infection Event. Jan 2023. Accessed at https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
- HSE Clinical Practice Guideline No. 12: Management of Early Pregnancy Miscarriage. Version 1.0, April 2012. Available from www.hse.ie

